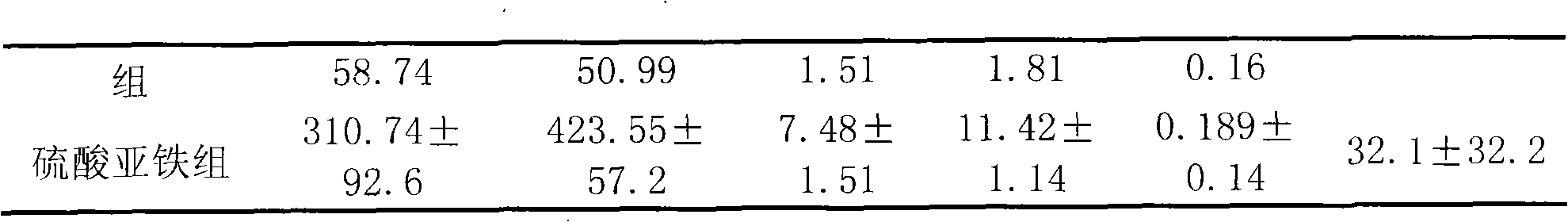Traditional Chinese medicine composition for treating iron deficiency anemia and preparation method thereof
A technology for iron deficiency anemia and a composition, which is applied in the field of pharmacy, can solve problems such as malabsorption of iron, and achieve the effects of less dosage, good stability and quick effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1. Granules of the present invention
[0042] Prescription: soap alum 4.5g, astragalus 270g, hawthorn 270g, new donkey-hide gelatin 90g, jujube 90g
[0043] Preparation method: Astragalus, hawthorn, and jujube are boiled twice with water for 2 hours each time, the amount of water added is 10 times and 8 times respectively, combined with the decoction, concentrated to a clear paste with a relative density of 1.08 (measured at 80 ° C), added Equal amount (calculated by volume) of 95% ethanol, stand for 48 hours or centrifuge, concentrate the supernatant or centrifuge to a clear paste with a relative density of 1.29-1.31 (measured at 80°C), and filter. Saponin and new donkey-hide gelatin are pulverized, and the fine powder is mixed with the above-mentioned clear paste, powdered sugar, and dextrin to make granules, that is, it is obtained. (1000g granules)
Embodiment 2
[0044] Embodiment 2, the drug effect test of the present invention
[0045] 1 Materials and methods
[0046] 1.1 Sample
[0047] Granules of the present invention: Example 1 of the present invention, the specification is 10g / bag; ferrous sulfate tablets, containing ferrous sulfate content of 0.3g / tablet, are provided by Jinan Yongning Pharmaceutical Co., Ltd., batch number 20051211.
[0048] 1.2 Experimental animals
[0049] 70 female weaned Wistar rats of SPF grade provided by the Laboratory Animal Center of Shandong Medical University were selected, weighing 50-60 g. The breeding environment is a barrier-level animal room, and the license number for the use of experimental animals is SYXK-(Lu) 2006-0025. Low-iron feed was provided by the Chinese Center for Disease Control and Prevention Nutrition and Food Safety.
[0050] 1.3 Dosage selection
[0051] The granules of the present invention are set with low and high dosage groups, which are respectively 0.5 and 1.0 g / kg.B...
Embodiment 3
[0087] Embodiment 3, clinical observation result report is as follows:
[0088] clinical information
[0089] A total of 224 cases of iron deficiency anemia were treated. Among them, there were 32 males and 192 females; the age ranged from 10 months to 79 years, with an average age of 36 years; the time since the onset of symptoms was 1 month to 22 years, and the average disease duration was 5.6 years.
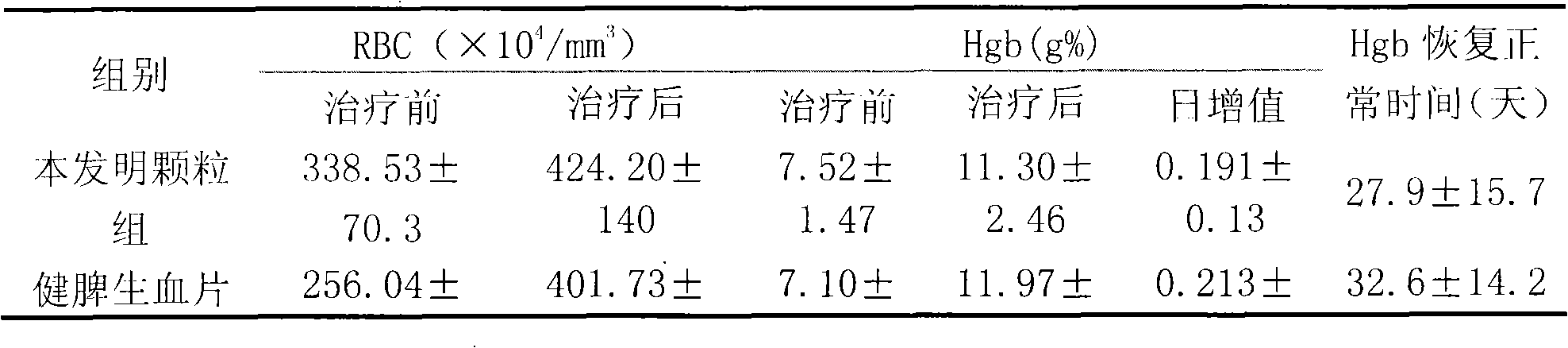
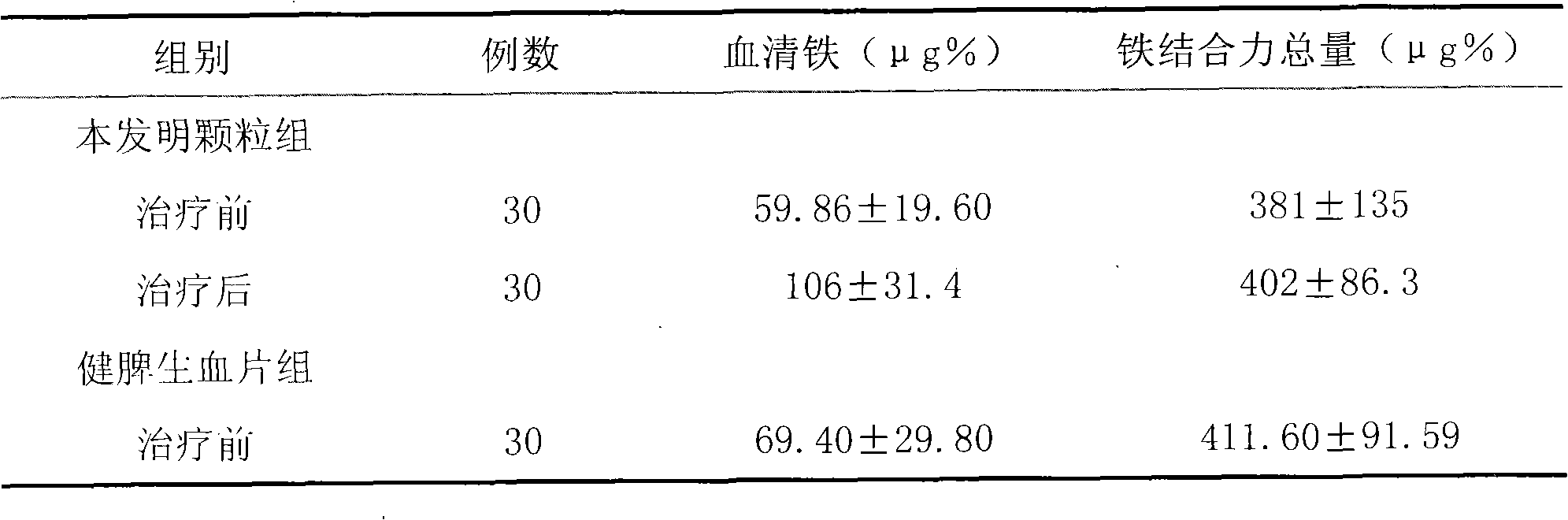
[0090] The 224 cases were divided into three groups for observation: 101 cases in the granule group of the present invention, 93 cases in the Jianpi Shengxue tablet group, and 30 cases in the ferrous sulfate group. A pre-selected observation form was filled out for each case before taking the medicine.
[0091] Treatment and Outcomes
[0092] 1. Treatment
[0093]1. Composition of medicines: Granules of the present invention: (Example 1 of the present invention) provided by Shandong Wohua Pharmaceutical Technology Co., Ltd., and the specification is 10g / bag; etc., produce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com