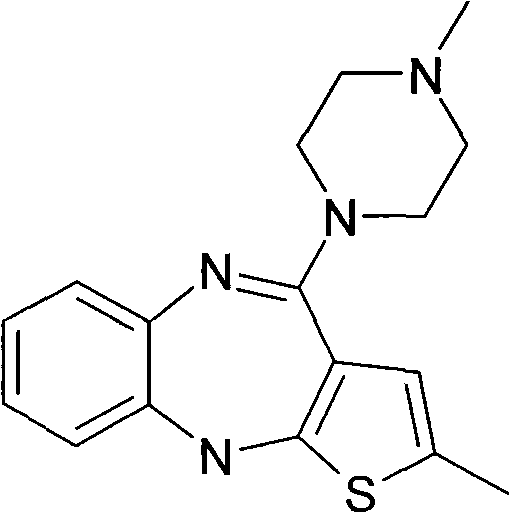
A kind of method for preparing olanzapine crystal form Ⅱ
A technology of olanzapine and crystal form, applied in organic chemistry and other directions, can solve the problems of prolonging drying time, high acetonitrile residue, reducing residual solvent, etc., to achieve the goal of reducing residual amount, good product purity, and conducive to market competition. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: Preparation of Olanzapine Form II
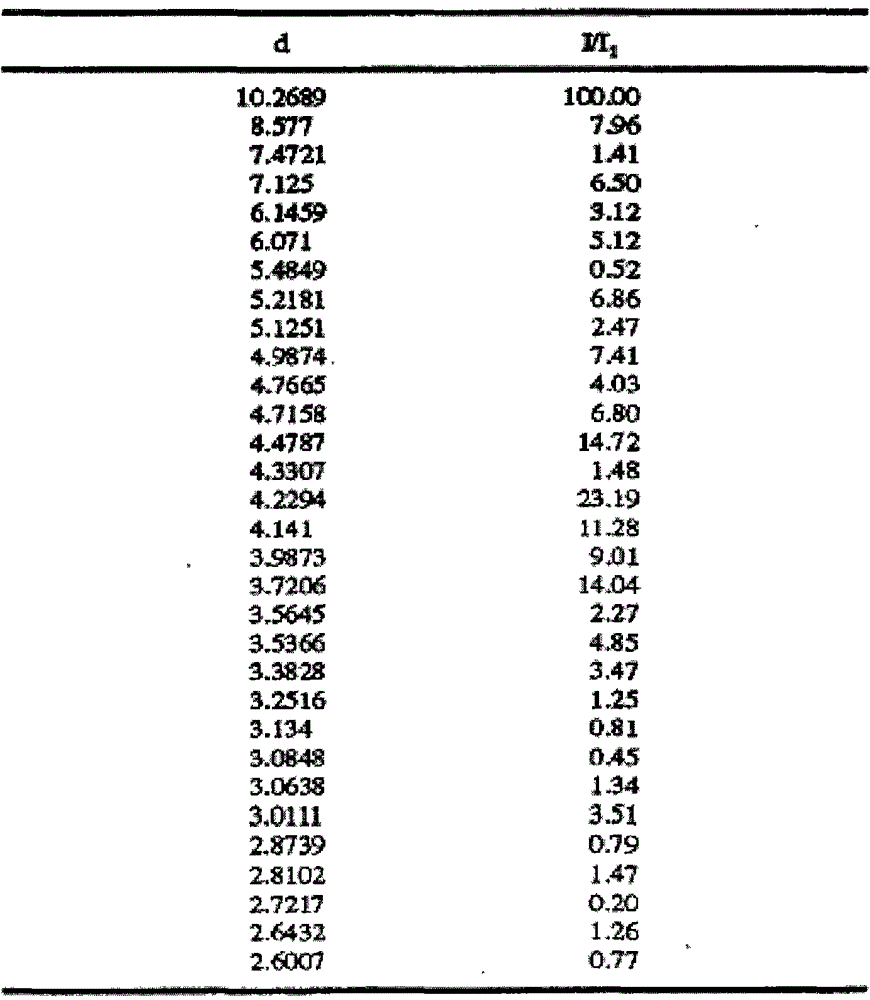
[0024] Add 10 g of crude olanzapine into 120 ml of acetonitrile, heat until dissolved, add 0.5 g of activated carbon, heat to reflux and keep stirring for 30 min under reflux. Filtrate while hot, slowly cool down the filtrate to 70°C and keep it warm at 65-70°C for 2 hours, then slowly cool it down to 55°C and keep it at 50-55°C and stir for 1.5 hours, then lower the temperature to 35°C within 1 hour and keep it warm for 30 Stir at ~35°C for 0.5 hours, finally cool down to 0-5°C and keep stirring for 1.5 hours, then filter. Add 30ml of ethyl acetate to the filter cake, heat to 60°C, stir and wash for 3 hours, then slowly cool down to 0-5°C and keep stirring for 1.5 hours, then filter. After the filter cake was dried, 9.1 g of solid was obtained, which was tested as olanzapine crystal form II, with a HPLC purity of 99.96%, 217 ppm of ethyl acetate and 255 ppm of acetonitrile.
Embodiment 2
[0025] Example 2: Preparation of Olanzapine Form II
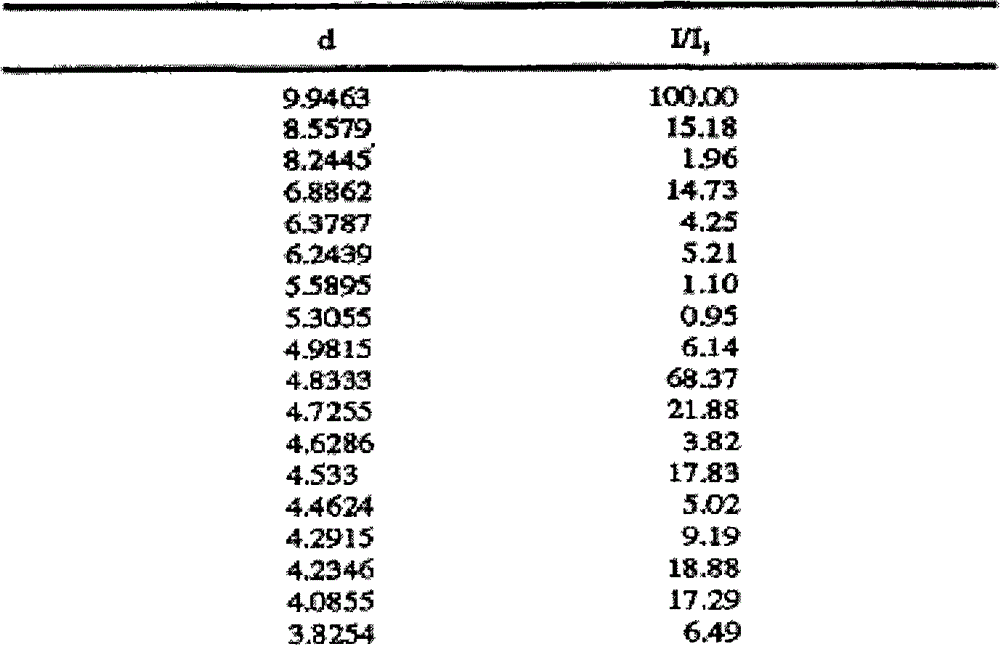
[0026] Add 10 g of crude olanzapine into 120 ml of acetonitrile, heat until dissolved, add 0.5 g of activated carbon, heat to reflux and keep stirring for 30 min under reflux. Filtrate while hot, slowly cool down the filtrate to 70°C and keep it warm at 65-70°C for 2 hours, then slowly cool it down to 55°C and keep it at 50-55°C and stir for 1.5 hours, then lower the temperature to 35°C within 1 hour and keep it warm for 30 Stir at ~35°C for 0.5 hours, finally cool down to 0-5°C and keep stirring for 1.5 hours, then filter. Add 30ml of toluene to the filter cake, heat to 60°C, stir and wash for 3 hours, then slowly cool down to 0-5°C and keep stirring for 1.5 hours, filter, and dry the filter cake to obtain 9.2g of solid, which is olanzapine crystal form after inspection II, HPLC purity 99.95%, residual toluene 209ppm, residual acetonitrile 305ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com