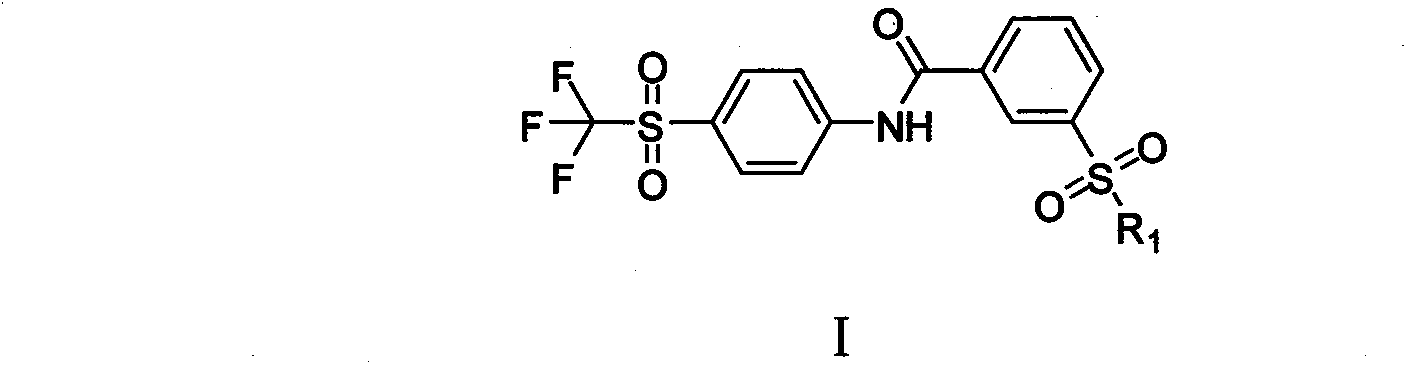
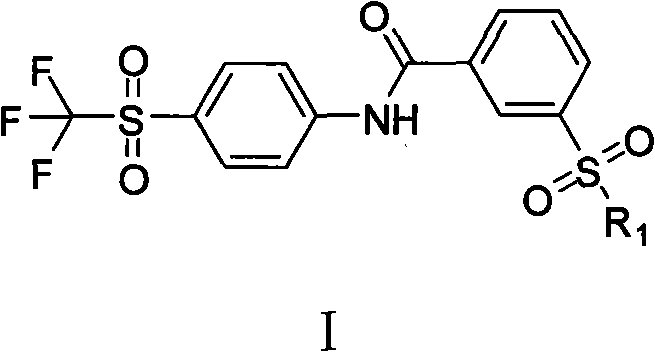
Soluble epoxide hydrolase inhibitor
An epoxide and hydrolase technology, applied in the fields of organic chemistry, drug combination, sulfonic acid amide preparation, etc., can solve the problems of poor water solubility, low bioavailability, limited druggability and clinical application, etc., and achieves a short synthetic route, Wide range of biological effects and less toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
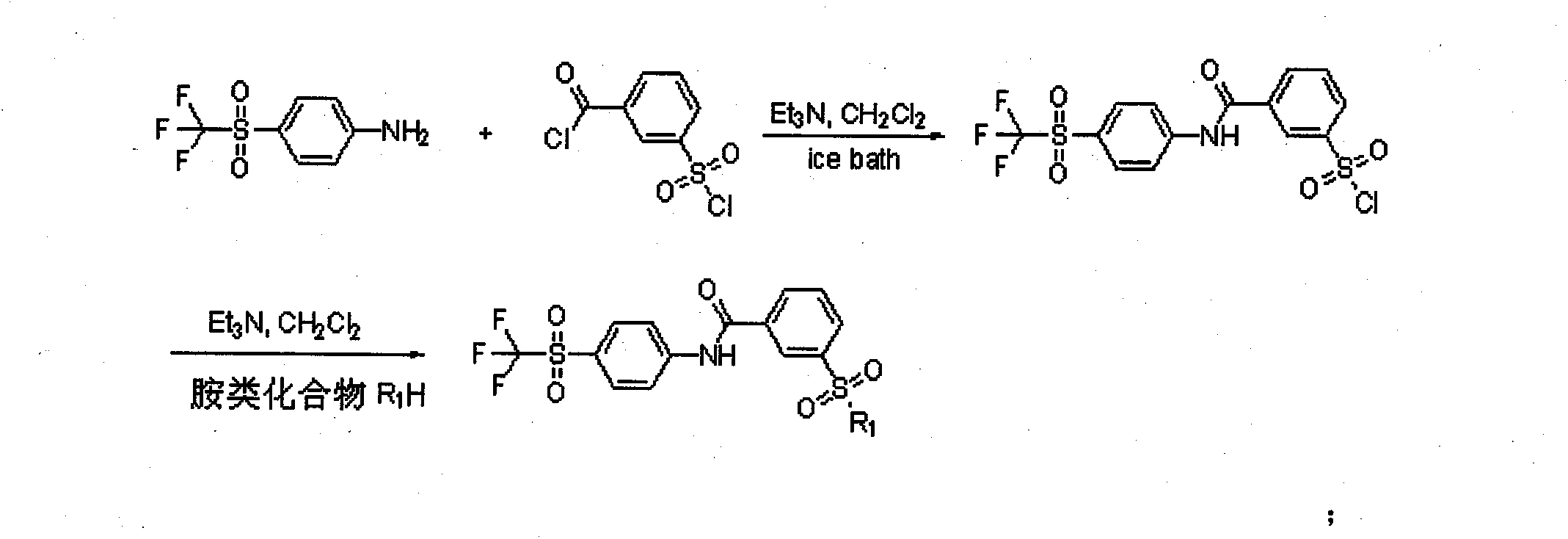
[0059] Synthesis of 3-(4-trifluoromethylsulfonyl)phenylcarbamoyl-1-sulfonyl chloride
[0060] In a 250mL three-necked flask, add 3-chlorosulfonylbenzoyl chloride (5.84g, 24.4mmol) into dry dichloromethane (16.6mL), stir in an ice bath at -10°C, and then add triethylamine ( 3.4mL, 24.4mmol) and 4-trifluoromethylsulfonanilide (5g, 22.2mmol), stirred overnight at room temperature to obtain a crude product.
Embodiment 2
[0062] Synthesis of 3-(morpholinylsulfonyl)-N-(4-(trifluoromethylsulfonyl)phenyl)benzamide (1a)
[0063] Take 2 mL of the crude product obtained in the previous step reaction, add it to 10 mL of dichloromethane, then add triethylamine (0.5 mL, 3.47 mmol) and morpholine (232 mg, 2.67 mmol) in sequence, continue the reaction at room temperature for 3 h, remove the solvent by rotary evaporation, and use silica gel The product was purified by column chromatography (PE:EA=3:1) to obtain 750 mg of white product with a yield of 71%. Product purity: 98%, 1 HNMR (ppm) 8.62-7.91 (m, 9H), 3.67 (t, 4H), 2.9 (t, 4H). MS (M + +1) 479, elemental analysis (%): calculated value C, 45.18; H, 3.58; F, 11.91; N, 5.85; O, 20.06; S, 13.40; found value C, 45.15; ; N, 5.87; O, 20.05; S, 13.41.
Embodiment 3
[0065] Synthesis of 3-(N-(2-methoxyethyl)aminosulfonyl)-N-(4-trifluoromethylsulfonyl)phenyl)benzamide (1b)
[0066] Take 2 mL of the crude product obtained in the previous step reaction, add it to 10 mL of dichloromethane, then add triethylamine (0.5 mL, 3.47 mmol) and 2-methoxyethylamino (200 mg, 2.67 mmol) successively, continue the reaction at room temperature for 6 h, spin The solvent was evaporated, and the product was purified by silica gel column chromatography (PE:EA=4:1) to obtain 800 mg of a yellow product with a yield of 77.3%. Product purity: 97.8%, 1 HNMR (ppm) 8.51-7.31 (m, 9H), 2.4 (br, 1H), 3.3 (t, 2H). 3.6 (t, 2H). 3.2 (s, 3H). MS (M + +1) 467, elemental analysis (%): calculated value C, 43.77; H, 3.67; F, 12.22; N, 6.01; O, 20.58; S, 13.75; found value C, 43.78; ; N, 6.02; O, 20.55; S, 13.73.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com