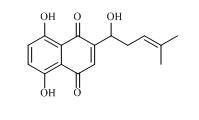
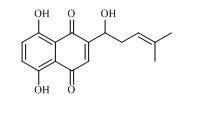
New application of hydroquinone compound
A hydroquinone and compound technology, which is applied to medical preparations containing active ingredients, digestive system, organic chemistry, etc., can solve the problem that the anti-HCV activity of the hydroquinone compound shikonin has not been reported yet, and achieve significant anti-hepatitis C resistance. Virus activity, extraction and separation methods are simple and easy, and the quality of the extract is good.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] A. Dried 20 kg Xinjiang comfrey ( A. euchroma ) After the stem bark is crushed, according to the mass / volume ratio (kg / L) of comfrey powder: ethanol aqueous solution = 1:2, fully mix 40 L of ethanol aqueous solution with a volume concentration of 95% and 20 kg of Xinjiang comfrey powder, Soak at room temperature for 24 hours to obtain an extract, repeat the extraction for 3 times, and combine the extracts;
[0021] B. Concentrate the extract in step A under reduced pressure as usual, recover ethanol until there is no ethanol smell, and then use petroleum ether to repeatedly extract the extract at room temperature according to the volume ratio of extract:petroleum ether=1:1 Second, after recovering the petroleum ether, the extract was obtained;
[0022] C. According to the volume ratio of extract: ethyl acetate=1:1, the extract of step B was repeatedly extracted 3 times with ethyl acetate at room temperature, and concentrated under reduced pressure to recover ethyl ace...
Embodiment 2
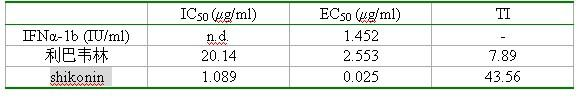
[0027] In this example, Huh 7.5.1 cells were infected with type 2a chimeric virus J6 / JFH1 to produce infectious virus particles, an HCV cell culture system (HCV cell culture, HCVcc), with interferon and ribavirin as positive controls , MTT cytotoxicity test and in vitro anti-HCV efficacy evaluation of anti-HCV drugs.
[0028] The compound shikonin was dissolved in DMSO, 0.22 mu M membrane filter sterilization, the concentration of the original drug solution was 10 mg / mL.
[0029] Human liver cancer cell line Huh 7.5.1 was treated with DMEM (Dulbecco’s modified Eagle’s medium, DMEM) containing 15% newborn bovine serum at 37°C and 5% CO 2 cultured in an incubator. Virus J6 / JFH1 was cultured from Huh 1 cells and stored at -80°C.
[0030] 1) MTT method to detect the cytotoxicity of samples
[0031] Huh 7.5.1 cells in the logarithmic growth phase were taken, and 9×10 3 cells / well cells were spread in 96-well plate, after 5 hours of attachment, add 2 mu L DMSO gradient dilu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com