Gemini surfactant and preparation method thereof
A technology of gemini surface and active agent, applied in the field of gemini surfactant and its preparation, anionic gemini surfactant and its preparation field, can solve the problems of difficulty, low yield, complex synthesis steps, etc., achieves easy operation and improved conversion High efficiency, easy purification effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
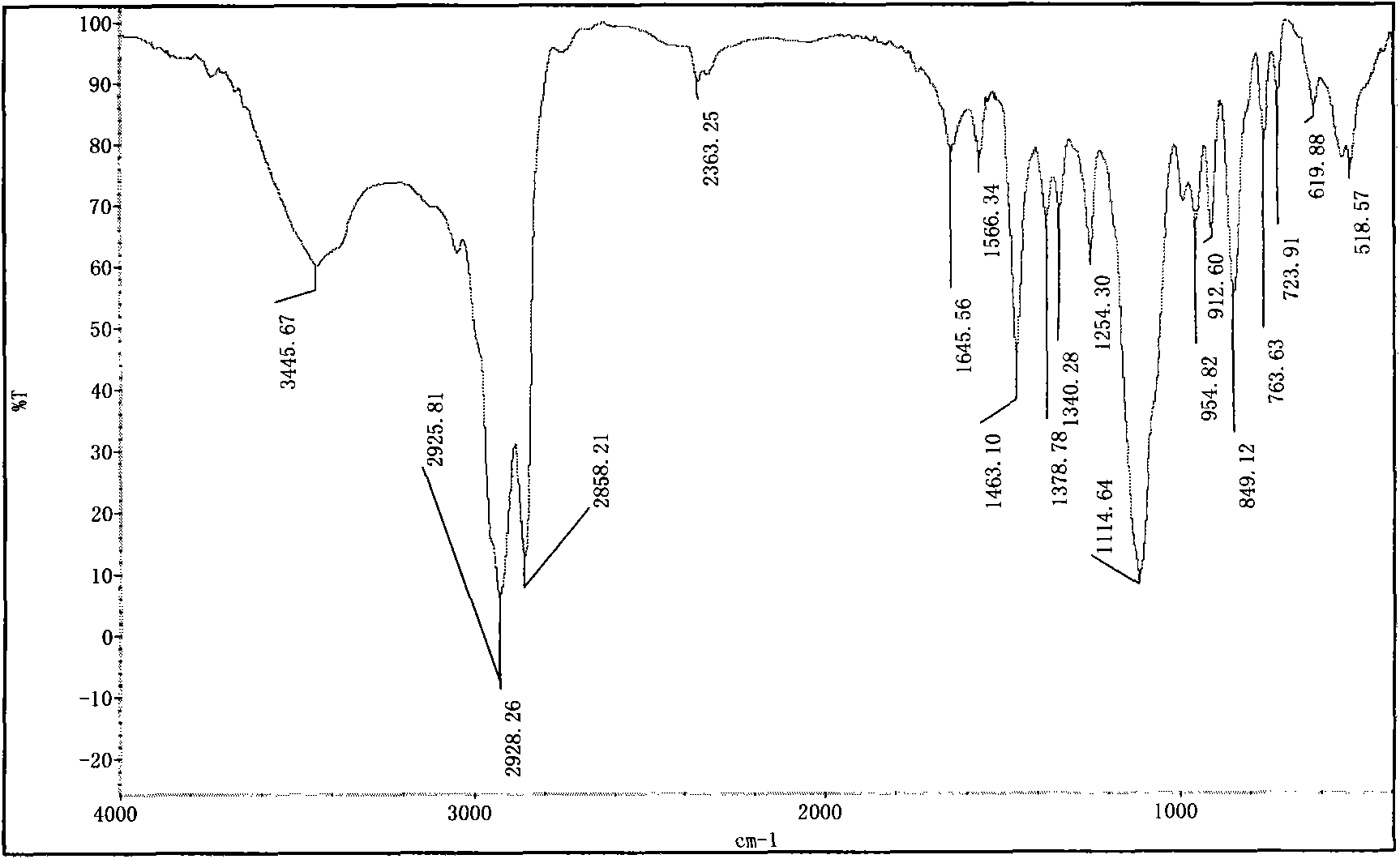
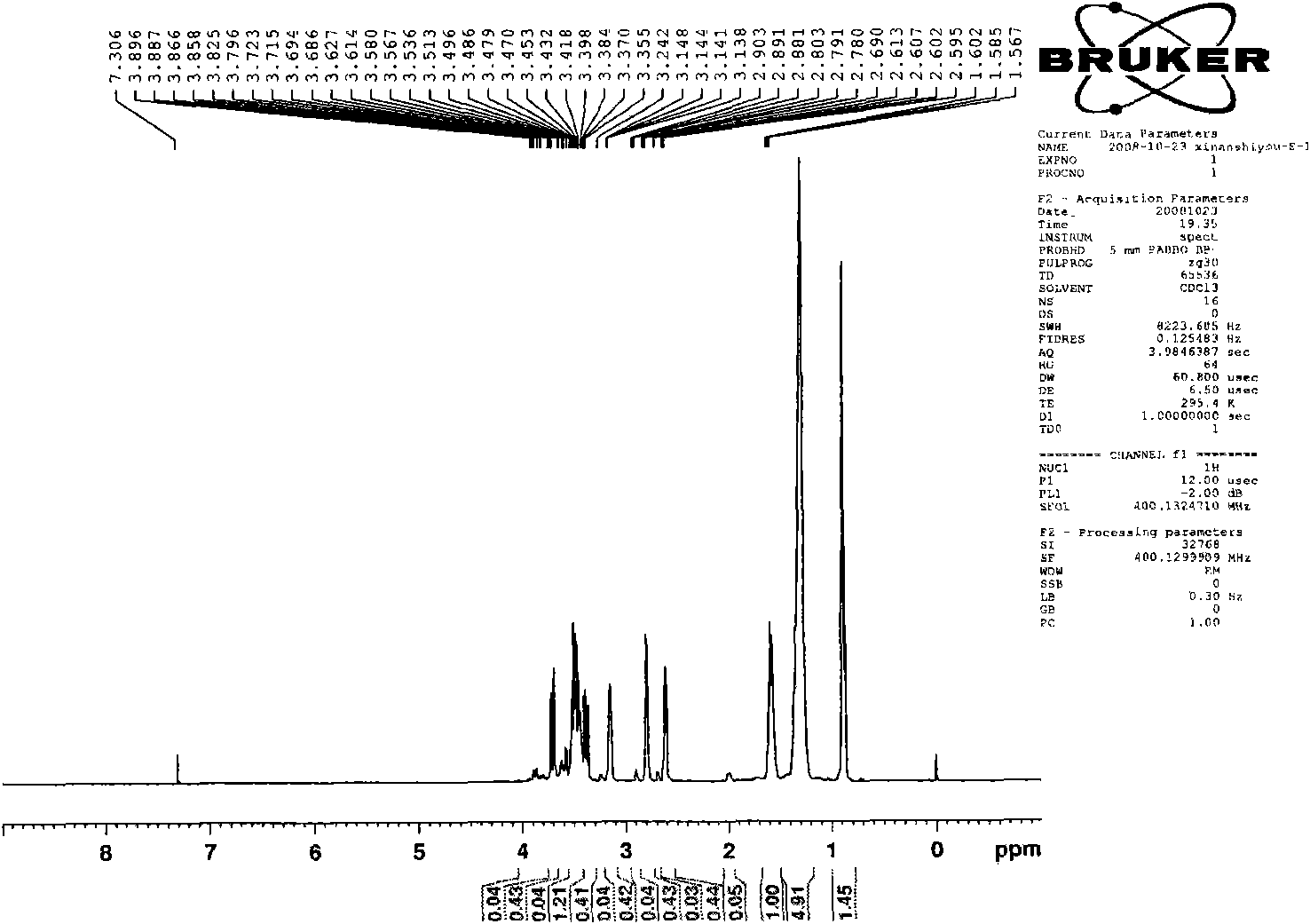
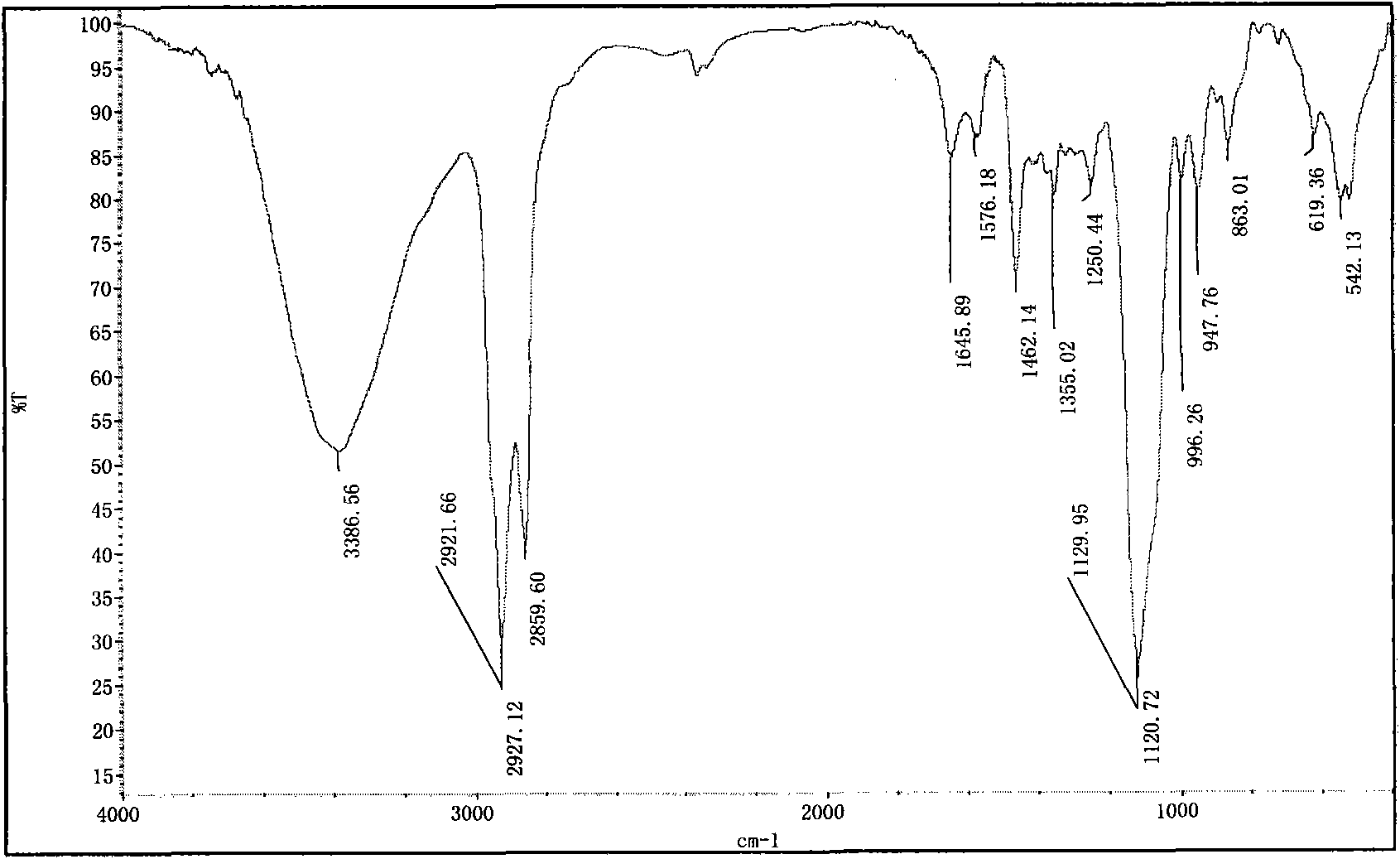
Image
Examples
Embodiment 1、1
[0059] The preparation of embodiment 1, 1,8-dioctylmethoxy-3,6-dioxa-1,8-tetracosane sodium disulfate (S-8-1-8)
[0060] (1) Preparation of n-octyl glycidyl ether (E-8)
[0061] Put a 250mL three-necked flask equipped with a thermometer and a constant pressure dropping funnel into a constant temperature magnetic stirring water bath, add 104.0g n-octanol, 48.0g sodium hydroxide, and 9.1g benzyltriethylammonium chloride into the flask, Stir and mix evenly at 30°C, slowly add 111g of epichlorohydrin (wherein the molar ratio of n-octanol, epichlorohydrin, sodium hydroxide and benzyltriethylammonium chloride is 1: 1.5: 1.35: 0.05 ), and keep warm for about 3 hours after the completion of the dropwise addition. After the reaction is completed, the product is suction filtered to remove sodium hydroxide and benzyltriethylammonium chloride, and then washed with water several times to combine the organic phase, and decompress at 0.005MPa and 95°C Water and unreacted epichlorohydrin wer...
Embodiment 2、1
[0066] Example 2, Preparation of 1,14-dioctylmethoxy-3,6,9-trioxa-1,14-sodium octadecanedisulfate (S-8-3-8)
[0067] (1) Preparation of n-octyl glycidyl ether (E-8)
[0068] The experimental procedure is the same as the preparation of (1) E-8 in Example 1; wherein, the basic compound used is potassium hydroxide, and the phase transfer catalyst is dodecyltrimethylammonium chloride, n-octanol, epichlorohydrin The molar ratio of potassium hydroxide and dodecyltrimethylammonium chloride is 1: 1.1: 1.1: 0.04, the reaction temperature is 35° C., and the reaction time is 4 hours.
[0069] (2) Preparation of 1,14-dioctylmethoxy-3,6,9-trioxa-1,14-octadecanediol (O-8-3-8)
[0070] Method is the same as the preparation of (2) O-8-1-8 in embodiment 1, triethylene glycol is replaced the ethylene glycol in the embodiment 1, wherein, E-8, triethylene glycol and potassium The molar ratio is still 1: 2: 0.1; its structural formula is as shown in formula (III), wherein, R is n-octyl, n is 1, ...
Embodiment 3、1
[0073] Embodiment 3, 1,8-two (dodecyl) methoxy-3, 6-dioxa-1, the preparation of 8-tridocosane sodium disulfate (S-12-1-12)
[0074] (1) Preparation of n-dodecyl glycidyl ether (E-12)
[0075] Method is with the preparation of (1) E-8 among the embodiment 1; Wherein, used fatty alcohol is n-dodecyl alcohol, and basic compound used is potassium hydroxide, and phase-transfer catalyst is tetrabutylammonium bromide, n-dodecyl alcohol The mol ratio of epichlorohydrin, potassium hydroxide and tetrabutylammonium bromide is 1: 1.1: 1.1: 0.04, and reaction temperature is 40 ℃, and the reaction time is 5 hours; Measure product epoxy value 6.06, theoretical ring The oxygen value is 6.58, and the product yield is 92%.
[0076] (2) Preparation of 1,8-bis(dodecyl)methoxy-3,6-dioxa-1,8-docosanediol (O-12-1-12)
[0077] Method is the same as the preparation of (2) O-8-1-8 in Example 1, E-12 is substituted for E-8 in Example 1, wherein, the mol ratio of E-12, ethylene glycol and potassium is ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com