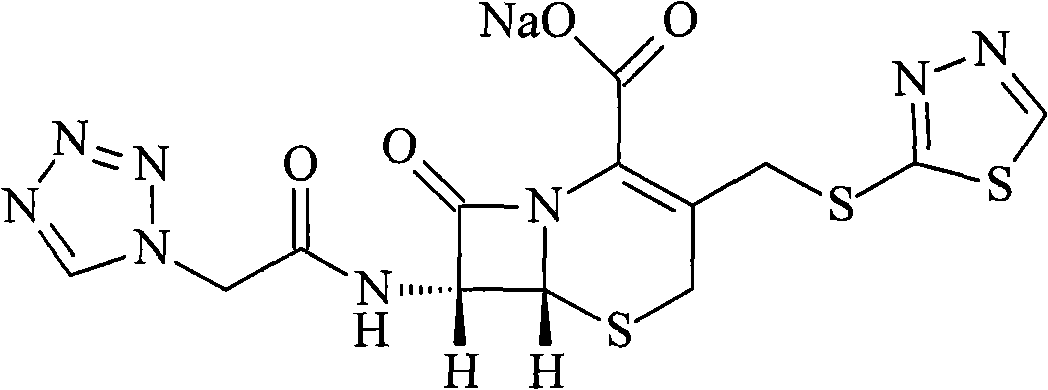
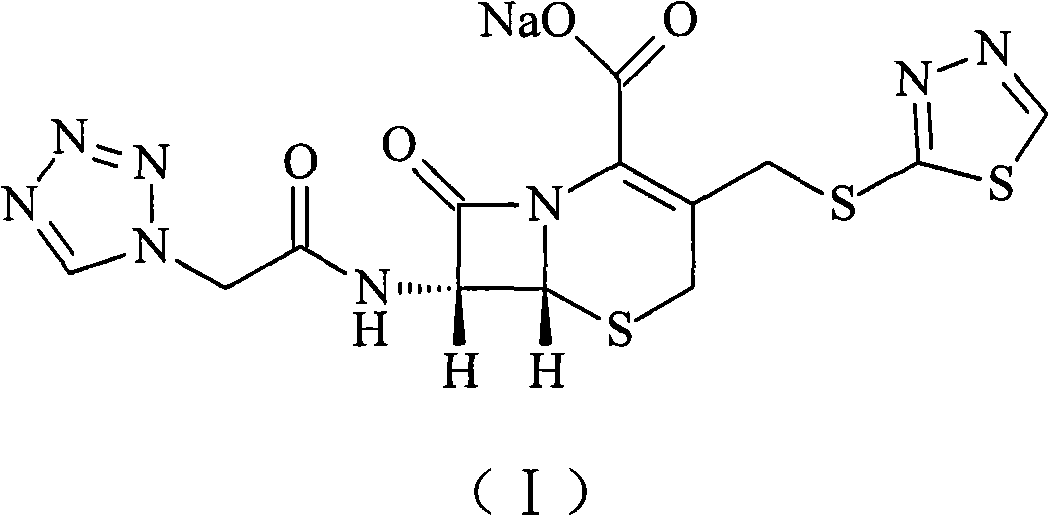
Ceftezole sodium compound and novel method thereof
A technology of ceftezole sodium and ceftezole, which is applied in the medical field, can solve the problems affecting the efficacy of clinical medication and the purity of ceftezole sodium is not very high, and achieve the effects of low cost, reducing toxic and side effects, and ensuring safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The refining of embodiment 1 ceftezole sodium
[0028] (1) 100g ceftezole sodium crude product is dissolved in 1000ml water, then slowly add the hydrochloric acid of 0.1mol / L, the pH of stirring reaction to solution is 1.5, promptly produces ceftezole precipitation, suction filtration obtains ceftezole 83.9g;
[0029] (2) 83.9g of ceftezole obtained in the previous step is dissolved in 400g of methanol, adding activated carbon with a total solution volume of 4.0g, insulated at 60°C and stirred for 30min, filtered for decarburization, and the filtrate is collected;
[0030] (3) The filtrate obtained in step (2) is processed using an anion / cation exchange membrane electrodialysis device: the anion exchange membrane uses the AHA anion exchange membrane provided by Japan Tokuyama Soda Company, and the cation exchange membrane uses Japan Tokuyama Soda Company to provide CMB cation-exchange membrane, feeds the solution that obtains by step (2) in the concentrated chamber, inj...
Embodiment 2
[0036] The refining of embodiment 2 ceftezole sodium
[0037] (1) 100g ceftezole sodium crude product is dissolved in 1000ml water, then slowly add the phosphoric acid of 0.5mol / L, the pH of stirring reaction to solution is 1.8, promptly produces ceftezole precipitation, suction filtration obtains ceftezole 82.1g;
[0038] (2) 82.1g of ceftezole obtained in the previous step was dissolved in 350g of methylene chloride, 3.5g of gac was added, stirred at 60° C. for 20 minutes, filtered for decarburization, and the filtrate was collected;
[0039] (3) The filtrate obtained in step (2) is treated with an anion / cation exchange membrane electrodialysis device: the anion exchange membrane uses the NEOSEPTA ACS anion exchange membrane produced by ASTOM Corporation, and the cation exchange membrane uses the NEOSEPTA CMX cation exchange membrane produced by ASTOM Corporation membrane, feed the solution obtained in step (2) into the concentration chamber, inject purified water into the i...
Embodiment 3
[0045] The refining of embodiment 3 ceftezole sodium
[0046] (1) 100g ceftezole sodium crude product is dissolved in 1000ml water, then slowly add the phosphoric acid of 0.5mol / L, the pH of stirring reaction to solution is 2.5, promptly produces ceftezole precipitation, suction filtration obtains ceftezole 80.9g;
[0047] (2) Dissolve 80.9g of ceftezole obtained in the previous step in 300g of isopropanol, add 3.0g of activated carbon, insulate at 60°C and stir for 30min, filter for decarburization, and collect the filtrate;
[0048] (3) The filtrate obtained in step (2) is processed using an anion / cation exchange membrane electrodialysis device: the anion exchange membrane uses the AHA anion exchange membrane provided by Japan Tokuyama Soda Company, and the cation exchange membrane uses Japan Tokuyama Soda Company to provide CMB cation-exchange membrane, feed the solution that obtains by step (2) in the concentrating chamber, inject purified water in the impurity-removing cham...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com