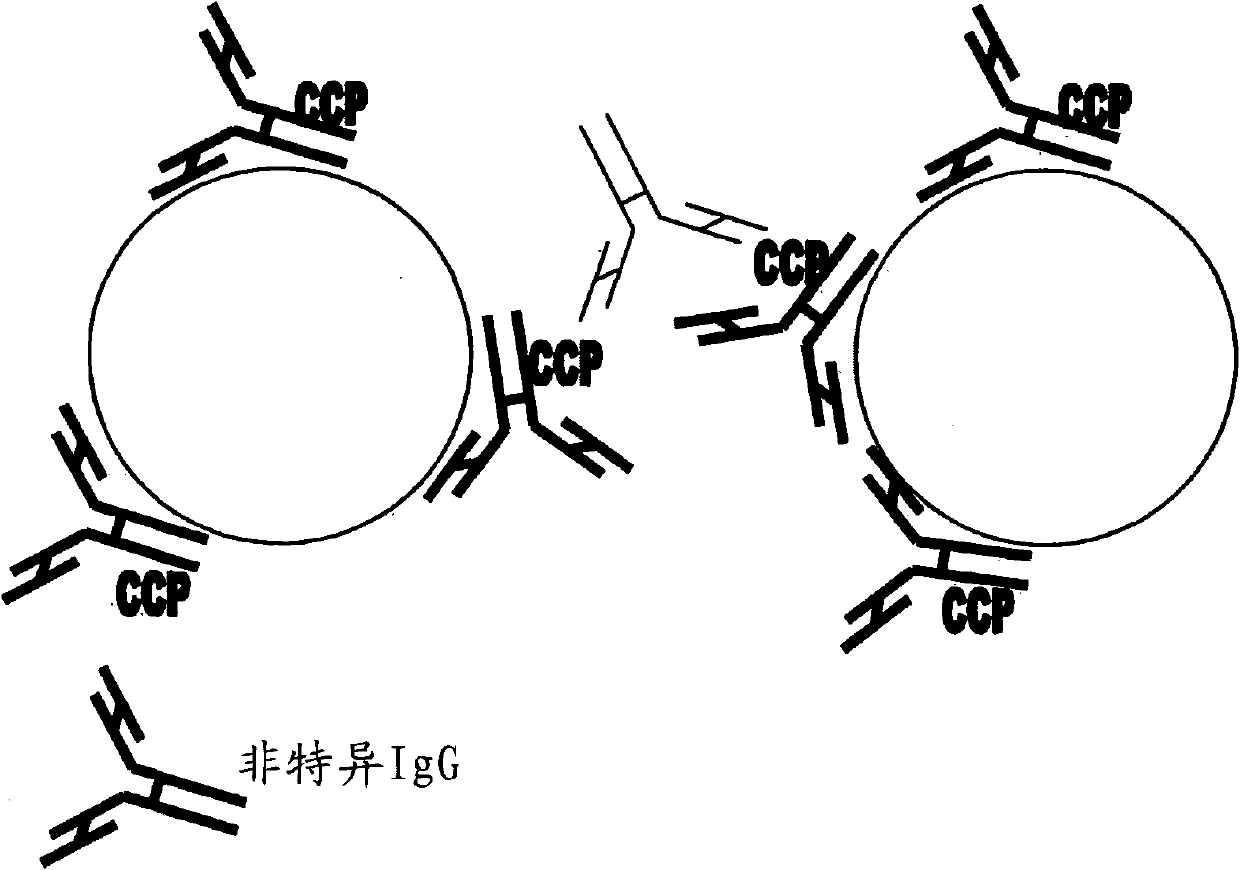
Assay method for antibodies against cyclic citrullinated peptide
An antibody and main body technology, applied in the field of detection of anti-cyclic citrullinated peptide antibodies, can solve the problems of difficult detection and low reliability, and achieve the effects of simple instrument use, cheap operation and high throughput
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Turbidity detection of anti-CCP antibody
[0085] (a) Preparation of avidin-coated nanoparticles
[0086] 600 μl of 4.2% w / v chloromethyl-activated nanoparticles (diameter 44 nm) (purchased from InterfacialDynamic Corporation, USA) were hydrodialyzed against a membrane with a pore size of 300,000 Da.
[0087] 0.5 ml of borate (10 mM) and sodium chloride (15 mM) solution (pH 9.0) was added and mixed. 10 mg of avidin dissolved in 0.5 ml of a 10 mM borate and 15 mM NaCl solution (purchased from Pierce Chemical Company) at pH 9.0 was added and the mixture was stirred at room temperature for 24 hours. Then 40 μl of a glycine solution (2M, pH 9.0) was added and the mixture was stirred for a further 4 hours at room temperature.
[0088] The microparticles were then diluted to a volume of 100 ml and dialyzed, first in 500 ml of 10 mM borate and 15 mM sodium chloride solution (pH 9.0), then in 25 mM Tris, 150 mM sodium chloride and 0.01% Tween 20 solution ( pH 7.4) (purchased...
Embodiment 2
[0093] Example 2: Turbidity Detection of Alternative Anti-CCP Antibodies
[0094] (a) Preparation of CCP peptide-coated nanoparticles
[0095] 1 ml of 4.2% w / v chloromethyl-activated nanoparticles (diameter 44 nm) (purchased from Interfacial Dynamic Corporation, USA) was subjected to water dialysis with a membrane having a pore size of 300,000 Da.
[0096] Then 0.5 ml of a 10 mM borate and 15 mM sodium chloride solution (pH 9.0) was added. 1.5 μg of purified CCP peptide (eg, affinity purified CCP peptide) was dialyzed against 10 mM borate and 15 mM sodium chloride solution (pH 9.0).
[0097] After the addition of nanoparticles to the purified CCP peptide, the mixture was stirred at room temperature for 24 hours. Then 40 [mu]l of glycine solution (2M, pH 9.0) was added and the mixture was stirred for a further 4 hours at room temperature.
[0098] The microparticles were then diluted to a total volume of 100 ml and dialyzed against 1000 ml of a 10 mM borate and 15 mM sodium ...
Embodiment 3
[0105] Example 3: Turbidity detection of anti-CCP antibody
[0106] (a) Preparation of streptavidin-coated nanoparticles
[0107] 600 μl of 4.2% w / v chloromethyl-activated nanoparticles (diameter 67 nm) (purchased from Interfacial Dynamic Corporation, USA) were subjected to water dialysis with a membrane having a pore size of 300,000 Da.
[0108] 0.5 ml of phosphate (10 mM) and sodium chloride (150 mM) buffer (pH 7.4) and 10 mg of streptavidin dissolved in 0.5 ml of 10 mM phosphate and 150 mM NaCl buffer (pH 7.4) (available from Pierce Chemical Company) were added together and the resulting mixture was stirred at room temperature for 24 hours. Then 40 [mu]l of glycine solution (2M, pH 9.0) was added and the resulting mixture was stirred for a further 4 hours at room temperature.
[0109] The microparticles were then diluted to a volume of 100 ml and dialyzed, first in 500 ml of 10 mM borate and 15 mM sodium chloride solution (pH 9.0), then in 25 mM Tris, 150 mM sodium chlori...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com