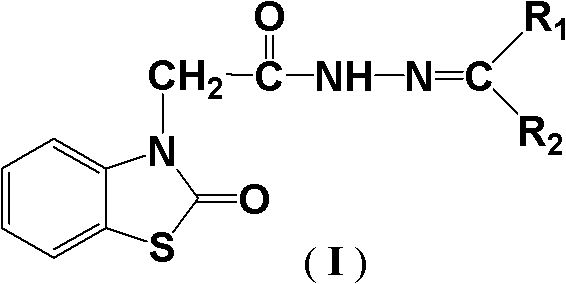
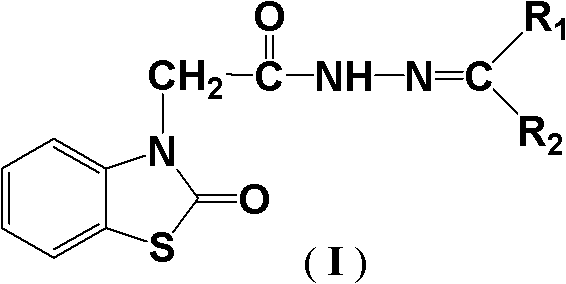
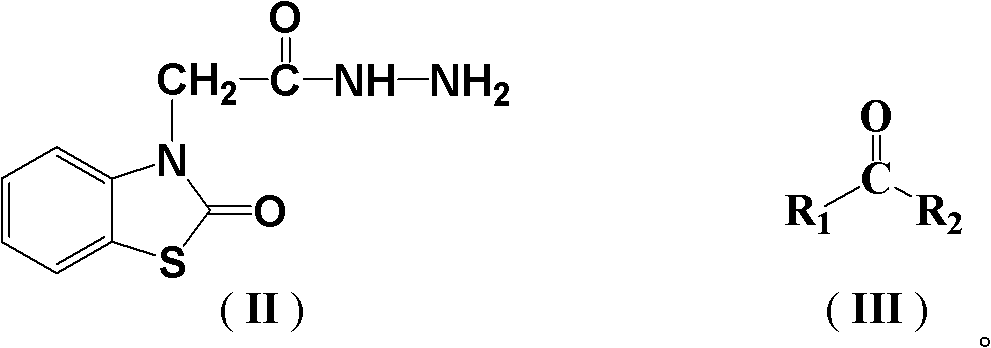(2-oxo-benzothiazole)-3-diacetyl hydrazone compound as well as preparation method and application thereof
A technology of benzothiazole and oxo generation, applied in the direction of botany equipment and methods, applications, chemicals for biological control, etc., can solve problems such as structure and preparation method that have not been reported, and achieve good insecticide and sterilization Active, easy to prepare effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1 (2-oxobenzothiazole)-3-acetylhydrazone compound I-1 (R 1 = Methyl, R 2 =Methyl) Synthesis
[0027] 2.25 g (10 mmol) (2-oxobenzothiazole)-3-acetylhydrazine and 0.58 g (10 mmol) acetone were dissolved in 20 mL of absolute ethanol, stirred to dissolve, heated to 78° C., and reacted under reflux for 1 hour. Following TLC tracking, the reaction was completed, the reaction solution was cooled and filtered, and the filter cake was recrystallized with 15 mL of ethyl acetate to obtain 2.39 g of white crystals, namely compound I-1. The melting point is 243~245°C and the yield (calculated as (2-oxobenzothiazole)-3-acetylhydrazine, the same below) is 90.7%.
[0028]
[0029] 1 The H NMR and IR spectrum analysis are as follows:
[0030] 1 H NMR(CDCl 3 )δ: 1.83(s, 3H, -CCH 3 CH 3 ), 2.04(s, 3H, -C CH 3 CH 3 ), 5.06(s, 2H, -CH 2 -), 6.91~7.44 (m, 4H, -Ph), 8.83 (s, 1H, -NH-);
[0031] IR v(cm -1 ): 3453, 3191, 3041, 1679, 1596, 1550, 1476, 1412, 1332, 1306, 1276, 1223, 1187, 10...
Embodiment 2
[0032] Example 2 (2-oxobenzothiazole)-3-acetylhydrazone compound I-2 (R 1 = Methyl, R 2 = Ethyl) Synthesis
[0033] Dissolve 2.25 g (10 mmol) (2-oxobenzothiazole)-3-acetylhydrazine and 1.08 g (15 mmol) butanone in 30 mL of absolute ethanol, stir to dissolve, heat to 78°C, and react under reflux for 1.5 hours. After the reaction was completed, the reaction solution was cooled and filtered, and the filter cake was recrystallized with 15 mL of petroleum ether to obtain 2.42 g of white crystals, namely compound 1-2. The melting point is 170-172°C, and the yield is 87.3%.
[0034]
[0035] 1 The H NMR and IR spectrum analysis are as follows:
[0036] 1 H NMR(CDCl 3 )δ: 1.14(t, 3H, J=7.5Hz, -CH 2 CH 3 ), 1.83(s, 3H, = C CH 3 C 2 H 5 ), 2.32~2.36(q, 2H, = CCH 3 CH 2 CH 3 ), 5.08(s, 2H, -CH 2 -), 6.92~7.47 (m, 4H, -Ph), 8.52 (s, 1H, -NH-);
[0037] IR v(cm -1 ): 3454, 3203, 3042, 2939, 1695, 1670, 1555, 1476, 1415, 1374, 1266, 1224, 1189, 1042, 986, 748.
Embodiment 3
[0038] Example 3 (2-oxobenzothiazole)-3-acetylhydrazone compound I-3 (R 1 = Methyl, R 2 =Phenyl) Synthesis
[0039] 2.25g (10mmol) (2-oxobenzothiazole)-3-acetylhydrazine and 1.32g (11mmol) of acetophenone were dissolved in 40mL of absolute ethanol, stirred to dissolve, heated to 78°C, and reacted under reflux for 2.5 hours. After the reaction was completed, the reaction solution was cooled and filtered, and the filter cake was recrystallized with 20 mL of n-hexane to obtain 2.80 g of white crystals, namely compound I-3. The melting point is 244-245°C, and the yield is 86.0%.
[0040]
[0041] 1 The H NMR and IR spectrum analysis are as follows:
[0042] 1 H NMR(CDCl 3 )δ: 2.20(s, 3H, =CPh CH 3 ), 5.23(s, 2H, -CH 2 -), 6.94~7.77 (m, 9H, -Ph), 9.12 (s, 1H, -NH-);
[0043] IR v(cm -1 ): 3451, 3192, 3036, 1694, 1663, 1556, 1474, 1420, 1366, 1333, 1265, 1235, 1178, 1103, 992, 763.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com