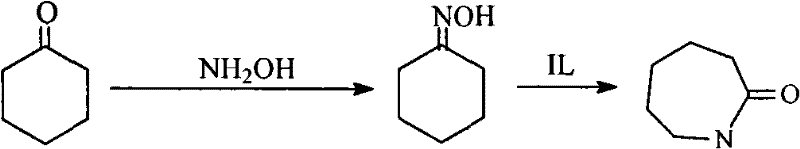
Process for directly synthesizing caprolactam from cyclohexanone and hydroxylamine
A technology of caprolactam and cyclohexanone, which is applied in the field of organic chemical synthesis, can solve the problems of energy consumption in the separation of intermediate products, and achieve the effects of improving effective utilization, reducing floor space, and not corroding equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Room temperature ionic liquid N, N, N-trimethyl-N-sulfobutyl-ammonium bisulfate is synthesized according to the method of Chinese patent, application number 200710151000.1:
[0024] In the first step, take an aqueous solution of trimethylamine containing 0.5 mol of trimethylamine and 0.5 mol of 1,4-butane sultone, put them in a 250 ml three-necked flask, and stir and react at room temperature for 12 hours.
[0025] In the second step, the reaction solution obtained in the first step is taken out and subjected to rotary evaporation to remove water to obtain a white zwitterionic solid.
[0026] In the third step, the substance obtained in the second step is washed successively with absolute ethanol, toluene and anhydrous ether, and then vacuum-dried to constant weight at 80 ° C to obtain the zwitterion N, N, N-trimethyl-N- sulfobutyl ammonium.
[0027] In the fourth step, take 0.5 mol of the white zwitterionic solid obtained in the third step and put it into a four-necke...
Embodiment 2
[0029] Dissolve 0.031mol of sodium nitrate and 0.028mol of sodium hydroxide in 10ml of water in a 250mL three-necked bottle, and then add 2.63mL of H 3 PO 4 solution (containing 0.045mol phosphoric acid), add water to make 15mL phosphate buffer solution, add hydroxylamine sulfate 0.006mol, cyclohexanone 0.012mol (1.26ml) to the buffer solution, and place the three-necked flask in a 50°C water bath for magnetic stirring reaction . After reacting for 30min, add 0.0366mol (10.7g) of the room temperature ionic liquid N,N,N-trimethyl-N-sulfobutyl-ammonium bisulfate prepared in Example 1, and stop the reaction after reacting for 20min under normal pressure . Extract with dichloromethane several times, each time using 5mL, combine the extracts, and analyze the extracts directly on a gas chromatograph. The reaction result is that the conversion rate of cyclohexanone is 12.4%, and the selectivity of caprolactam is 84.2%.
Embodiment 3
[0031] The steps are the same as in Example 2, except that the reaction is stopped after adding the room temperature ionic liquid N,N,N-trimethyl-N-sulfobutyl-ammonium bisulfate prepared in Example 1 for 30 minutes. As a result of the reaction, the conversion rate of cyclohexanone was 13.2%, and the selectivity of caprolactam was 89.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com