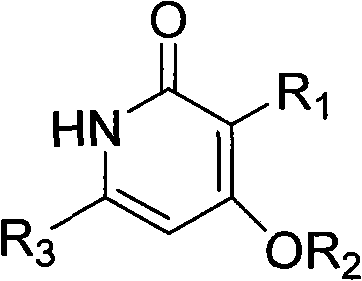
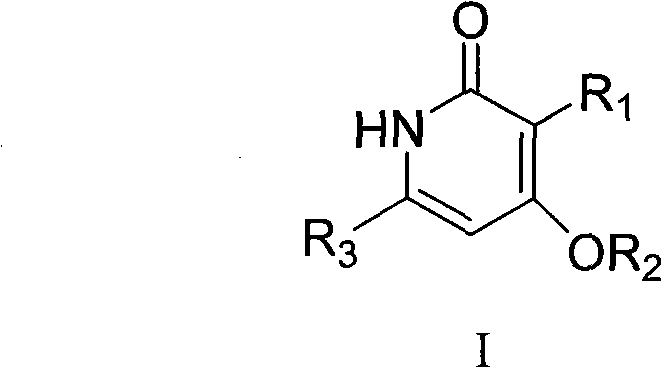
Preparation and application of novel pyridone human immunodeficiency virus-1 (HIV-1) reverse transcriptase inhibitor
A technology of pyridone and CH20H, which is applied in antiviral agents, medical preparations containing active ingredients, organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Embodiment 1: the preparation of isopropylidene malonate (compound 1)
[0016] Dissolve 52g (0.5mol) of malonic acid in 60ml (0.6mol) of acetic anhydride, add 1.5ml of concentrated sulfuric acid under ice water cooling and stirring, add dropwise 40ml (0.55mol) of acetone after 10min, stir at room temperature for 20min, and dissolve the mixture Place in the refrigerator overnight, filter under reduced pressure to obtain a white solid, recrystallize in acetone-ice water, and the yield is 72.2%. mp: 95-96°C (literature value 94-95°C).
Embodiment 2
[0017] Example 2: Preparation of 3-oxo-4-phenyl-butyric acid ethyl ester (compound 2a)
[0018] Add 5.45g (37.81mmol) isopropylidene malonate, 70ml dichloromethane, 6.2ml pyridine to a 100ml round-bottomed flask, add 7ml (52.93) phenylacetyl chloride dropwise after cooling in ice water for 15min, and continue cooling for 1h. Stir overnight. The reaction solution was washed with 10% HCl aqueous solution (50ml*2), washed with water (50ml), anhydrous Na 2 SO 4 dry. Filter and evaporate to dryness under reduced pressure to obtain a red-black solid, add 100ml of absolute ethanol to the solid, heat to reflux for 4h, spin to dry the solvent, petroleum ether: ethyl acetate = 50:1 and purify to obtain the target product 2a, light yellow oil, yield: 56.75%.
[0019] 1 H NMR (300MHz, CDCl 3 )δ: 7.20-7.37 (m, 5H, Ar-H), 4.16 (q, 2H, OC H 2 CH 3 ), 3.83(s, 2H, CH 2 -Ph), 3.51(s, 2H, CH 2 ), 1.26(t, 3H, OCH 2 CH 3 ).
[0020] Preparation of 3-oxo-4-(4'-fluorophenyl)-butyric ...
Embodiment 3
[0037] Example 3: Preparation of ethyl 4-hydroxy-6-benzyl-2(1H)-pyridone-3-carboxylate (compound 3a)
[0038] Add 61ml (60.68mmol) of 2M ammonia methanol solution to 5g (24.27mmol) of 3-oxo-4-phenyl-butyric acid ethyl ester, seal the upper end of the reflux tube with a balloon, heat to reflux for 6h, and spin dry the solvent for later use.
[0039] In 40ml of absolute ethanol, add 1.2g of sodium metal, cool down after the reaction is complete, add dropwise 7.4ml (48.8mmol) diethyl malonate, the solution becomes cloudy, heat to reflux for 2h, after cooling, dropwise add 3-oxo- The ethanol solution after ammonolysis of 4-phenyl-butyric acid ethyl ester was heated to reflux for 3 days, and a large amount of white precipitate appeared. After the solution was cooled, a large amount of solid appeared when poured into 1N HCl, filtered through a Buchner funnel, and washed with ethyl acetate to obtain the target product. It is a white solid, and the raw material is recycled. Yield: 25...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com