Application of 5- (3'-Indolyl)-oxazole compound in preparing drugs for inhibiting mycobacterium tuberculosis and curing tuberculosis
A technology of Mycobacterium tuberculosis and oxazoles, which is applied in the direction of antibacterial drugs, etc., can solve the problems that are difficult to penetrate the cell wall and limit clinical application.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1. The fermentation culture of Streptomyces 9792
[0037] 1) Composition of medium
[0038] a. ISP2 slant medium: malt extract 1.0%, yeast extract 0.4%, glucose 0.4%, agar 1.2%, pH 7.0.
[0039] b. Primary and secondary seed medium: glucose 0.5%, malt extract 1.0%, yeast extract 0.5%, cottonseed cake powder 1.0%, KH 2 PO 4 0.05%, (NH4) 2 SO 4 0.5%, CaCO 3 0.3%, NaCl0.1%, pH7.2.
[0040] 2) Culture method
[0041] Cultivate Streptomyces 9792 on the ISP2 slant medium at 28°C for one day; inoculate the slant of the strain on the primary seed medium and cultivate it at 28°C for 2 days; transfer the primary seed medium to the secondary seed at 5% The culture medium was cultured at 28°C for 4 days, and the fermentation broth was harvested.
Embodiment 2
[0042] The separation and purification of embodiment 2.2-methyl-5-(3'-indolyl) oxazole and 2-ethyl-5-(3'-indolyl) oxazole
[0043]25 liters of Streptomyces 9792 fermentation broth was filtered, and the filtrate was adsorbed with DiaionHP20 macroporous adsorption resin at a volume ratio of 10:1, followed by a gradient of water, 20%, 50%, 80%, and 100% acetone of two column volumes For elution, the activity of each eluate was followed by disc agar diffusion. After testing, the active component is in the 80% acetone eluent, and the diameter of its bacteriostatic zone is about 28mm. The fraction eluted with 80% acetone was collected and concentrated under reduced pressure to obtain 6.8 g of brown-red crude product. Weigh 1 gram of crude product, put it on a 30mmI.D.×400mm ODS column, and elute with 30%, 60%, 80%, and 100% methanol-water solution gradient respectively, collect and measure activity in sections, and the active components are washed in 60% methanol During dehydratio...
Embodiment 3
[0044] Physical and chemical properties and spectral characteristics of embodiment 3.2-methyl-5-(3'-indolyl) oxazole and 2-ethyl-5-(3'-indolyl) oxazole
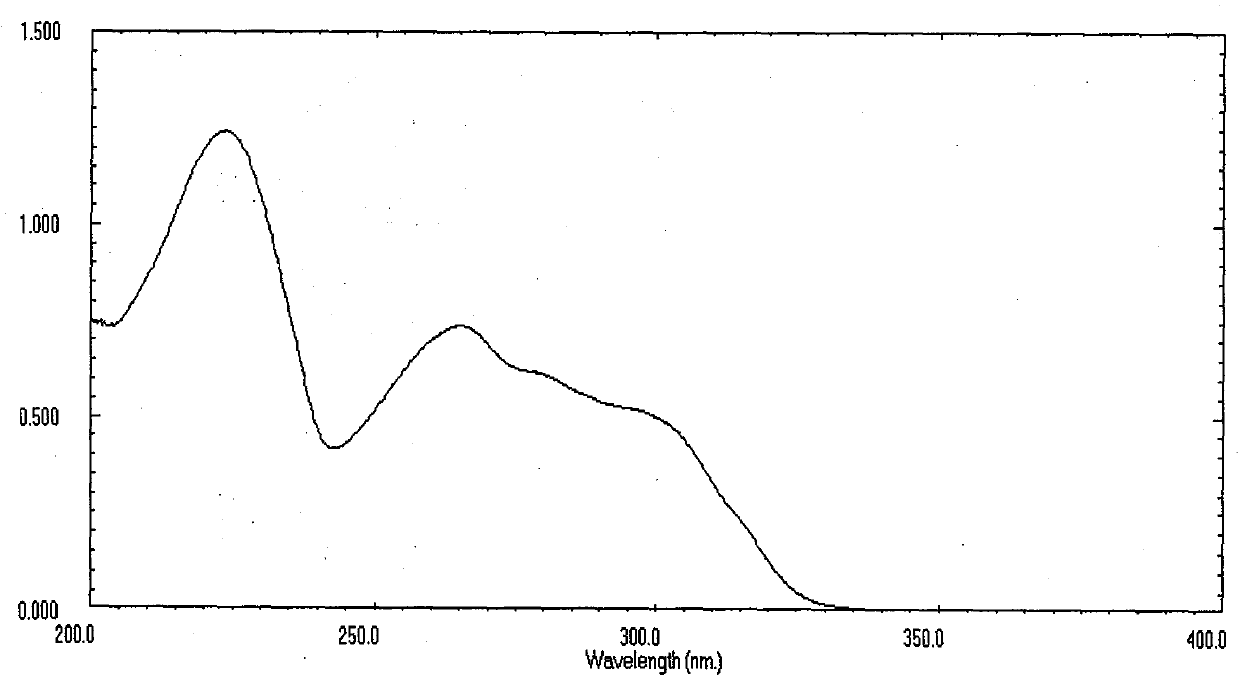
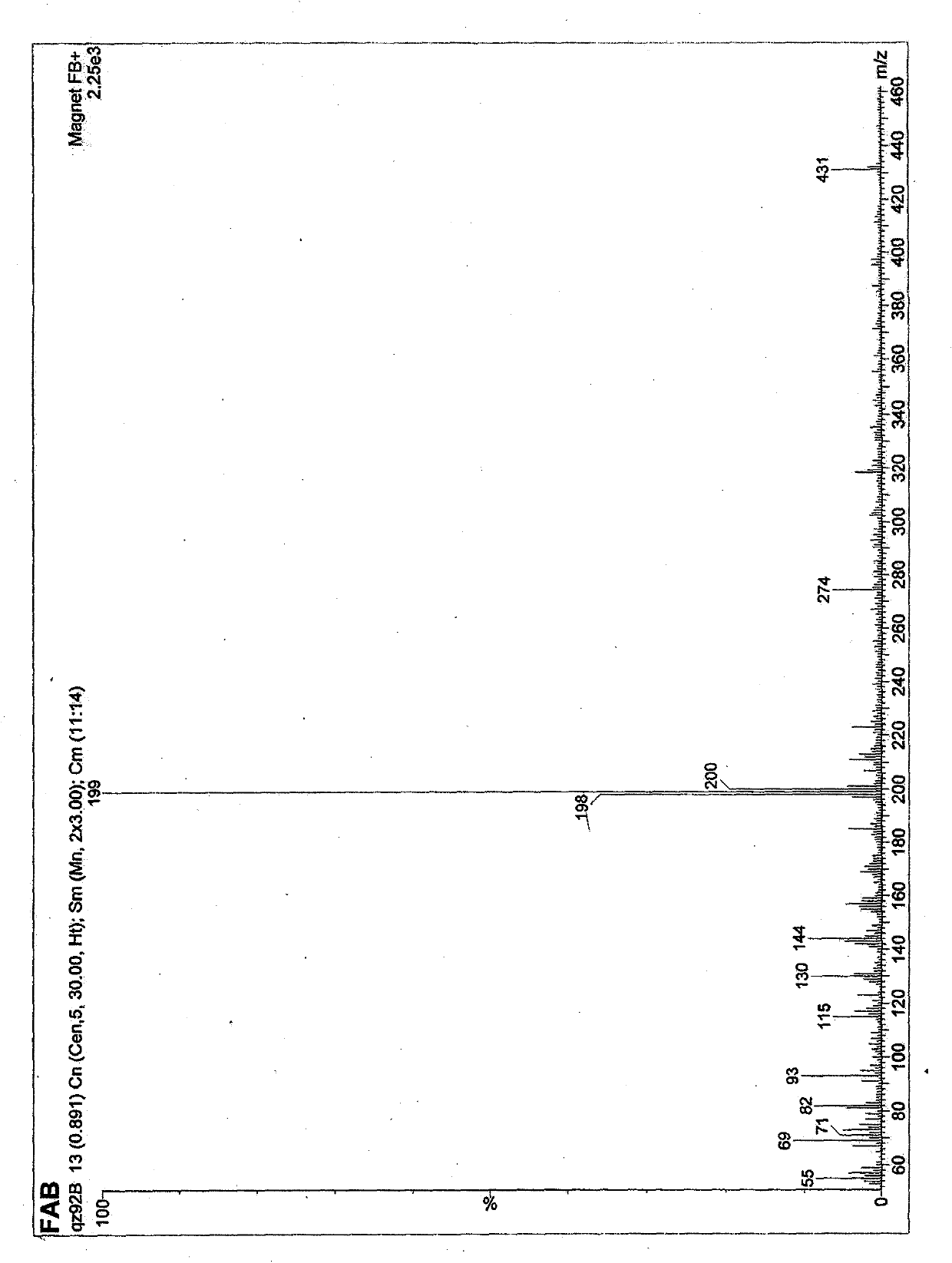
[0045] 2-Methyl-5-(3'-indolyl)oxazole is a white powder, easily soluble in methanol, ethyl acetate, chloroform and other organic solvents, For 224, 265, 282 (sh), 298 (sh). Molecular weight is 198, molecular formula C 12 h 10 N 2 O.
[0046] 2-Ethyl-5-(3'-indolyl)oxazole is a white powder, easily soluble in methanol, ethyl acetate, chloroform and other organic solvents, For 224, 265, 282 (sh), 298 (sh). The molecular weight is 212, and the molecular formula is C 13 h 12 N 2 O.
[0047]
[0048] 2-Methyl-5-(3'-indolyl)oxazole R=CH 3
[0049] 2-Ethyl-5-(3'-indolyl)oxazole R=CH 2 CH 3
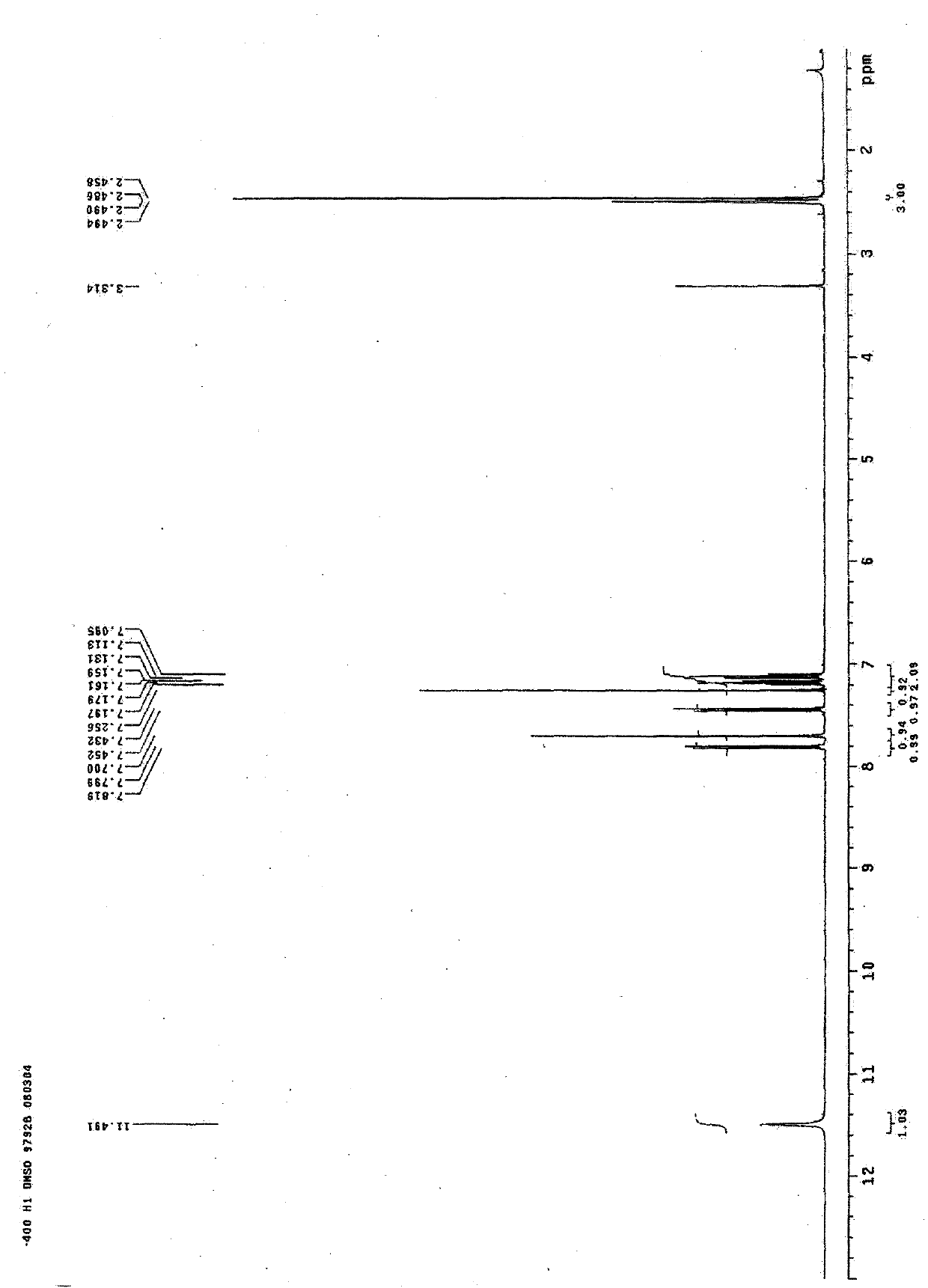
[0050] Table 1 H and C spectra data of 2-methyl-5-(3’-indolyl)oxazole and 2-ethyl-5-(3’-indolyl)oxazole
[0051]
[0052] a: 500M Hz in CD3OD b: 125M Hz in CD3OD c: 400M Hz in DMSO-d6 d: 100MHz in DMSO-d6
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com