Benzofurane, benzothiophene, benzothiazol derivatives as fxr modulators
A compound, C1-C6 technology, applied in anti-inflammatory agents, drug combinations, non-central analgesics, etc., can solve problems such as risk reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0457] The process for the preparation of the compounds of formula (I) according to the invention is another object of the invention.
[0458] According to a first aspect, compounds of formula (I) according to the invention can be prepared according to a process comprising reacting a compound of formula (II):
[0459]
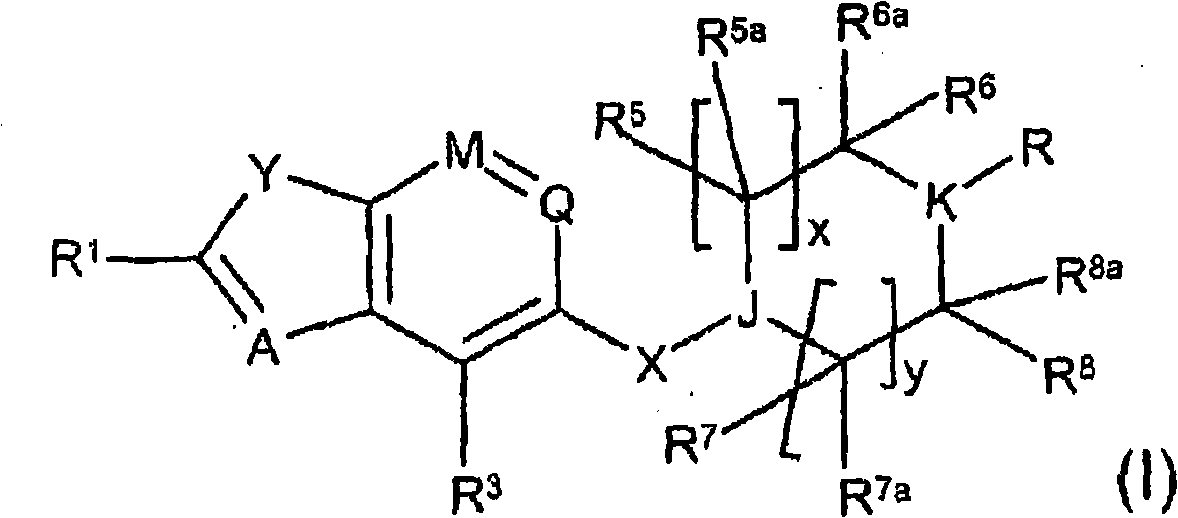
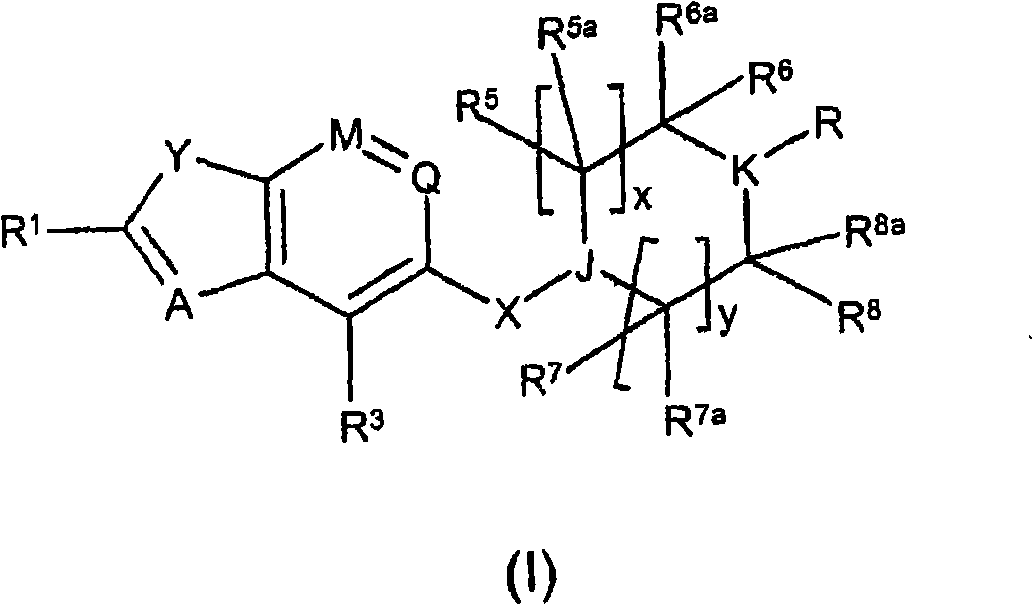
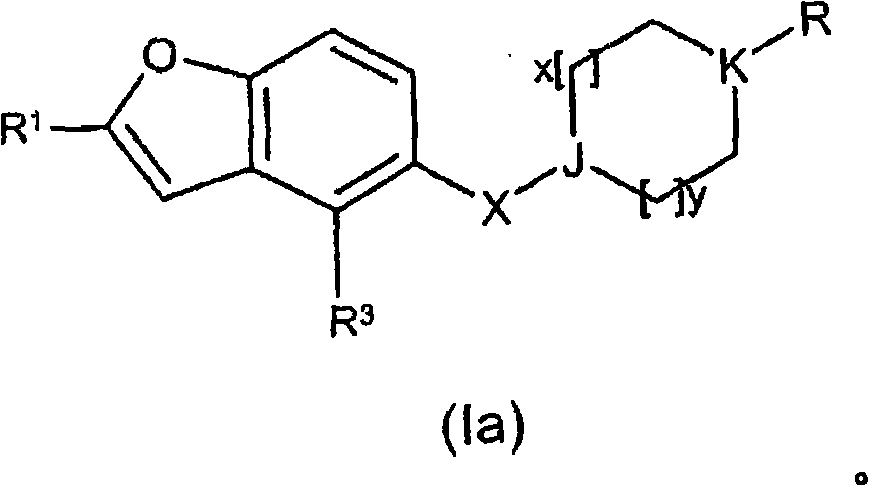
[0460] where R 1 , Y, A, M, Q, R 3 , X, J, R 5 , R 5a , R 6 , R 6a , R 7 , R 7a , R 8 , R 8a , x and y are as defined in formula (I), U is H or an amine protecting group.
[0461]"Amine protecting group" refers to a readily removable group known in the art that is capable of protecting an amino group from unwanted reactions and that can be selectively removed during synthetic procedures. The use of amine protecting groups as protecting groups to avoid undesired reactions during synthesis is well known in the art, and a variety of such protecting groups are known, for example, from T.H. Greene and P.G.M. Wuts, Organic Synthesis Protective Groups in...
Embodiment approach
[0464] According to a first embodiment, compounds of formula (II) can be prepared by reacting compounds of formula (III) with compounds of formula (IV):
[0465]
[0466] where R 1 , Y, A, M, Q, R 3 , X, J, R 5 , R 5a , R 6 , R 6a , R 7 , R 7a , R 8 , R 8a , x, y and U are as defined in formula (II), and Hal is a halogen atom.
[0467] Often, especially in the presence of palladium catalysts, such as Pd(OAc) 2 The aromatic substitution of the Hal group of the compound of formula (III) by the compound of formula (IV) is carried out in the presence of and BINAP.
[0468] In a particular aspect, compounds of formula (III) can be prepared according to a process comprising reacting compounds of formula (V):
[0469]
[0470] where M, Q, R 3 , Hal as defined in formula (III), Alk is C 1 -C 6 alkyl.
[0471] In general, the cyclization of compounds of formula (III) is carried out in the presence of a suitable base, for example an alkali metal alcoholate, such as ...
Embodiment 1
[0509] Example 1: {5-[4-(2,6-dichlorobenzyl)piperazin-1-yl]-4-methyl-benzofuran-2-yl}-morpholin-4-yl-methanol ketone
[0510]
[0511] 2-methoxy-6-methylbenzaldehyde
[0512]
[0513]Potassium peroxodisulfate (89.31g, 0.33mol) and copper (II) sulfate pentahydrate (27.22g, 0.11mol) were added to 2,3-dimethylanisole (15g, 0.11mol) in acetonitrile water mixture (750ml , 1:1) solution, the reaction mixture was stirred under reflux for 15 min until no raw material was detected by TLC (thin layer chromatography), the reaction mixture was cooled to room temperature, the product was extracted with dichloromethane (2 × 225ml), and water (2×100ml) and brine solution (100ml) washed the organic layer, dried the organic layer over sodium sulfate, and concentrated under reduced pressure to obtain the crude product. Column chromatography, eluting with 4 / 96 ethyl acetate / hexane, gave 2 - Methoxy-6-methylbenzaldehyde (7.32 g, 44.2%). 1 H NMR (300MHz, CDCl 3 ), δ2.60 (3H, s), 3.90 (3H...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com