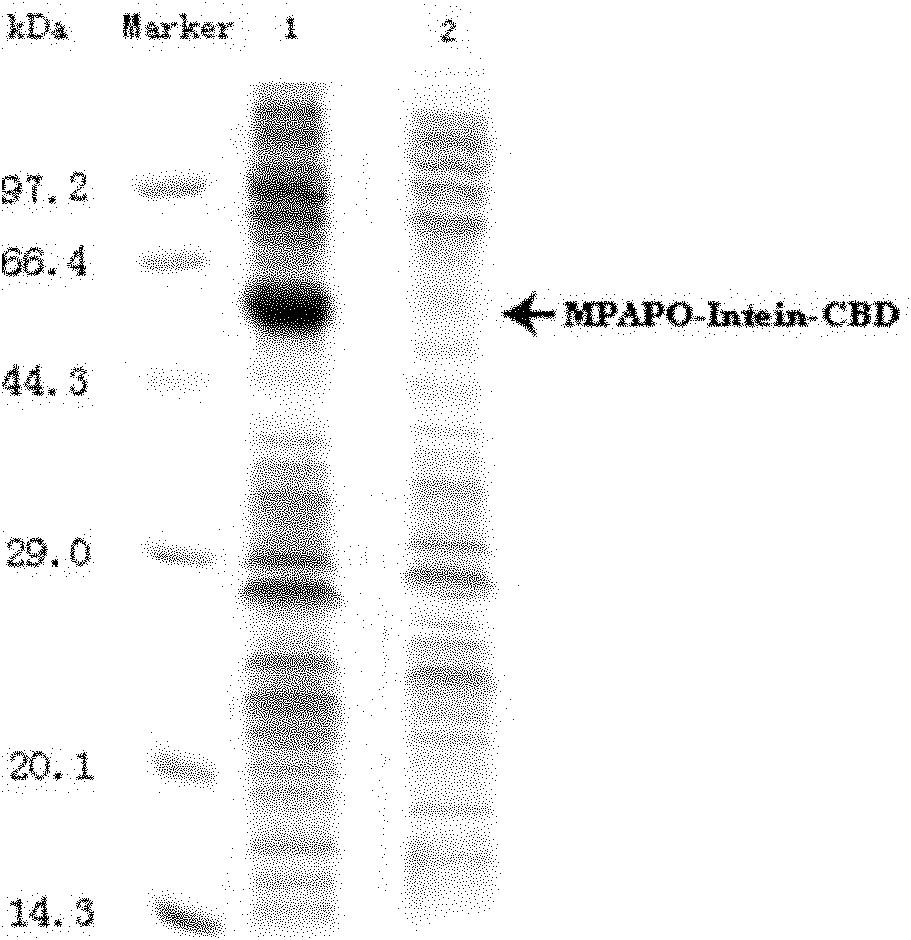High-stability PAC1 type receptor specific agonist MPAPO and preparation method and application thereof
A receptor-specific, high-stability technology, applied in peptide preparation methods, chemical instruments and methods, DNA/RNA fragments, etc., can solve the problems of loss of activity and poor stability, and achieve the promotion of nerve regeneration, low cost, and heart Significant effect in vascular disease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
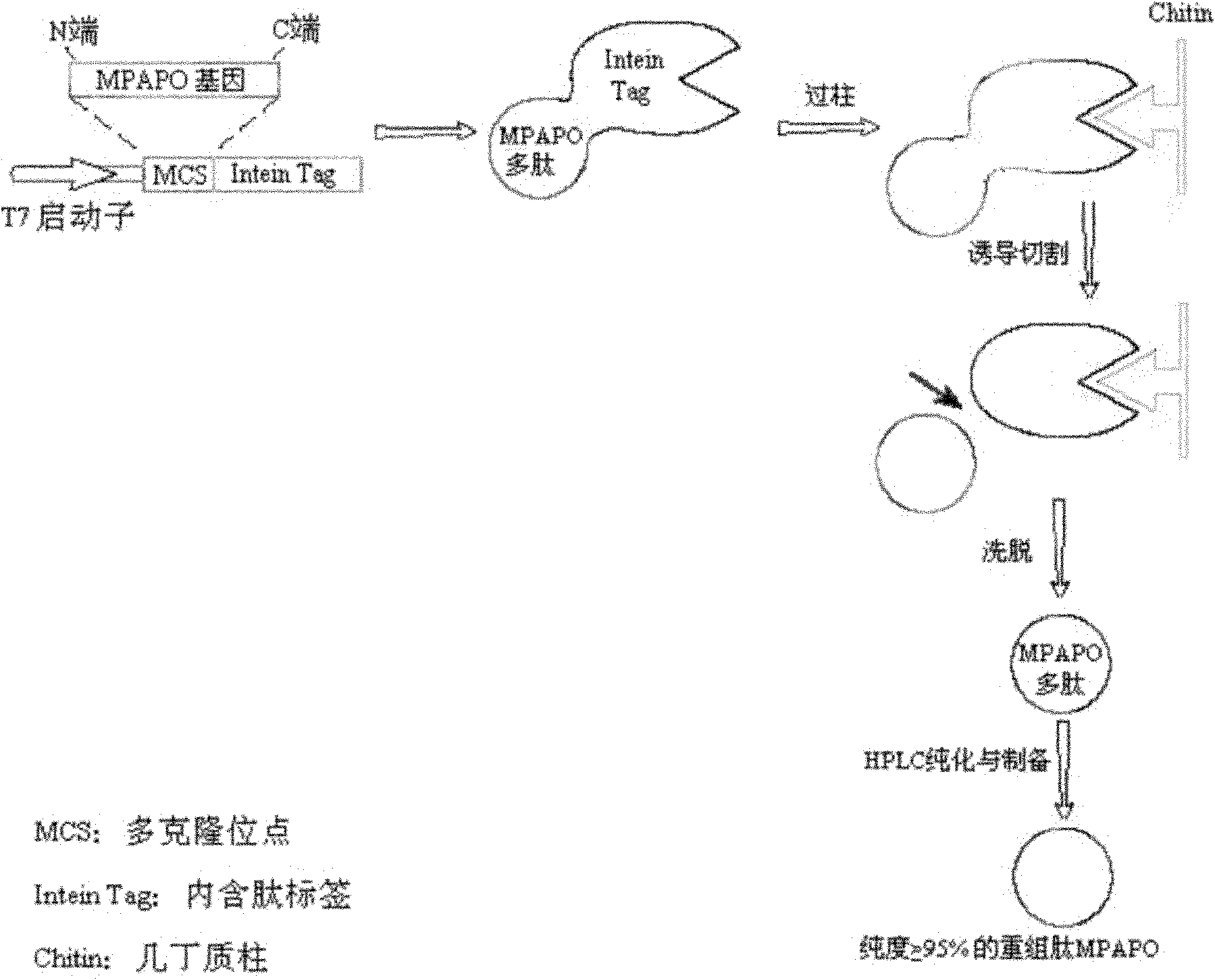
[0060] The preparation process of high stability PAC1 type receptor specific agonist MPAPO is as follows: figure 1 As shown, the specific steps are as follows:
[0061] (1) Obtaining of MPAPO coding sequence:
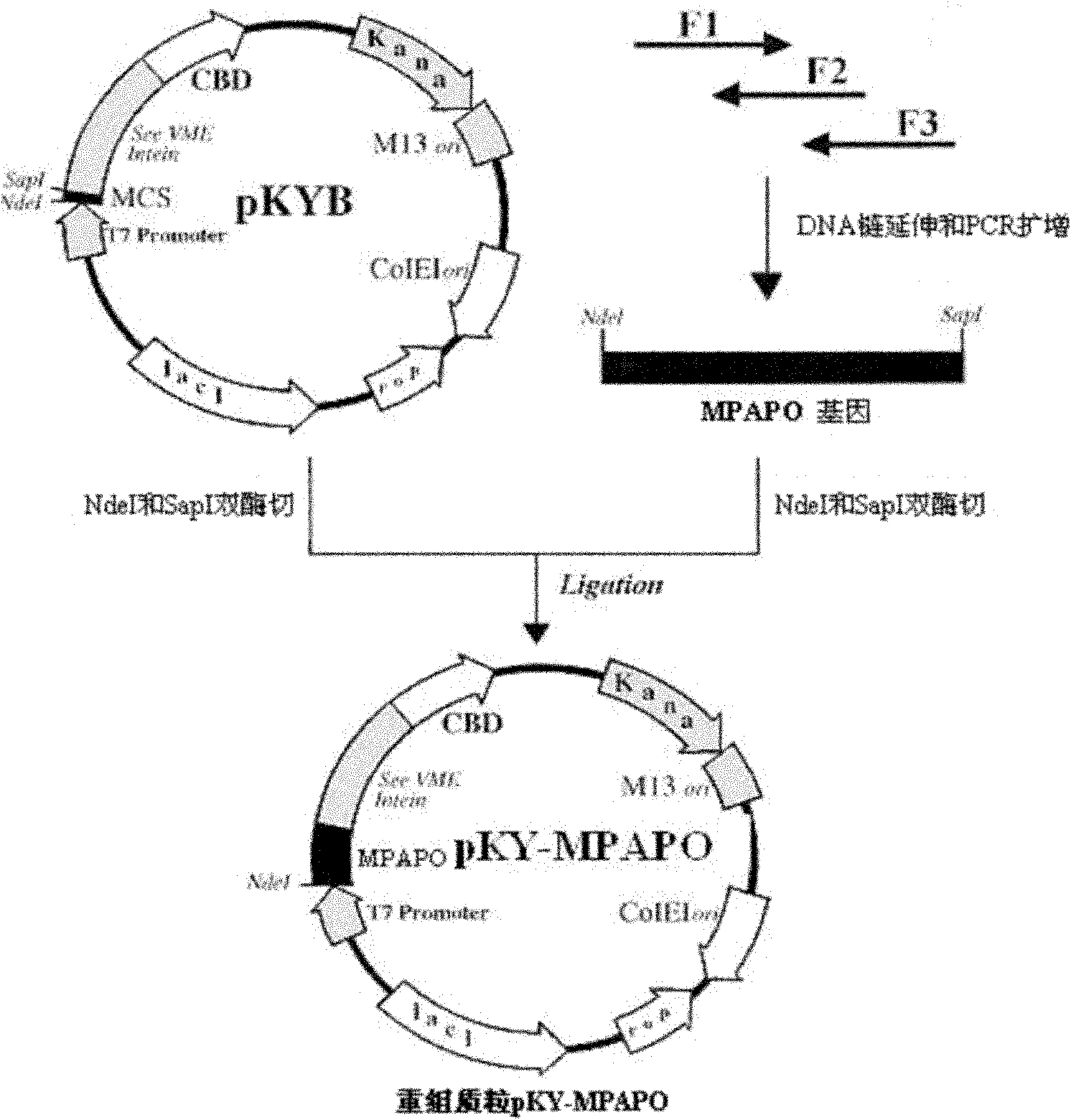
[0062] Design the cDNA encoding MPAPO according to the coding preference of Escherichia coli, design 3 primers, and adopt the two-step method to obtain the sequence (such as figure 2 shown):
[0063] Primer F1: 5'- GGT GGT CATATG CAT AGC GAT GGC ATT TTT ACC GAT AGC TAT AGC-3';
[0064] Primer F2: 5'-TTT TTT CAC CGC CAG CTG TTT GCG ATA GCG GCT ATA GCT ATC GGT-3';
[0065] Primer F3:
[0066] 5'- CCACCA TGCTCTTCCGCA TTT TTT CAC CGC CGC CAG ATA TTT TTT CAC CGC CAG-3’
[0067] in, GGT GGT or CCACCA To protect the base, CATATG is the NdeI restriction site, and TGCTCTTCCGCA is the SapI restriction site;
[0068] ① Chain extension reaction:
[0069] The reaction system is: 2 μL of primer F1 (250 μM / L), 2 μL of primer F2 (250 μM / L), 10×TaKaRaBuffer (containing 4...
Embodiment 2
[0085] The in vitro stability determination of recombinant peptide MPAPO (the MPAPO that is embodiment 1 preparation)
[0086] Recombinant peptide MPAPO, chemically synthesized MPAPO (compared with recombinant peptide MPAPO, its N-terminal has no methionine) and wild-type PACAP38 (product of US Biological Company) were dissolved in 20mM sodium phosphate buffer (pH 8.0, containing 150mM sodium chloride), incubated in a 37°C water bath, sampling at different time points, and using liquid chromatography-mass spectrometry (LC-MS) to detect the retention of the polypeptide in the aqueous environment over time, and Determine the stability of the recombinant peptide MPAPO in an aqueous solution environment in vitro, the results are as follows Figure 5 Shown: When incubated for 1 week, wild-type PACAP38 was reduced by 53.2%, when incubated for 2 weeks, wild-type PACAP38 was reduced by 79.1%, when incubated for 3 weeks and 4 weeks, wild-type PACAP38 was reduced by 85.5% and 96.0%, res...
Embodiment 3
[0088] Determination of Competitive Binding Activity of Recombinant Peptide MPAPO to PAC1 Receptor
[0089] PAC1-CHO cells (Invitrogen Company) were inoculated into 12-well plates for culture, and 2 h before the experiment, the cells were washed twice with 0.5 mL of serum-free Ham's F-12 medium. Then, the cells were cultured overnight at 4°C with Ham’s F-12 medium containing 2% (mg / ml) bovine serum albumin (BSA) and 10 mM glucose, and 0.1 nM [ 125 I] PACAP38 and the polypeptide to be tested (being wild-type PACAP38 and recombinant peptide MPAPO), and gradually increase the polypeptide concentration to be tested (10 -12 M~10 -5 M). After culturing, discard the supernatant, wash the cells 3 times with ice-cold PBS (0.01M, pH 7.4), mix the cells with 0.5mL 0.5M NaOH and 0.1% (mg / ml) SDS at room temperature for 10min, and then The radioactivity of cell lysates was measured by gamma counting method, and the half-inhibitory concentration (IC50, half-maximal inhibitory concentrati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com