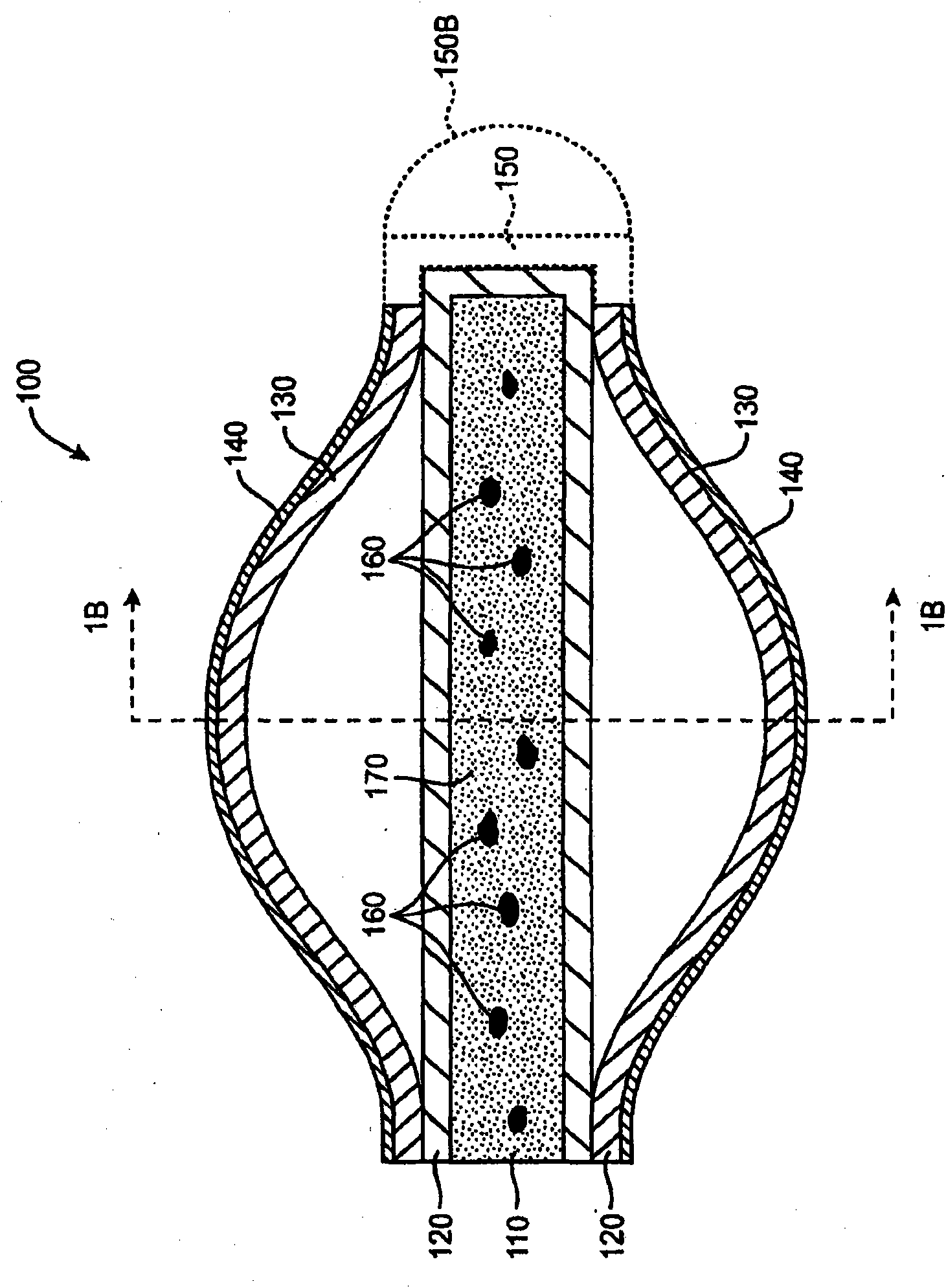
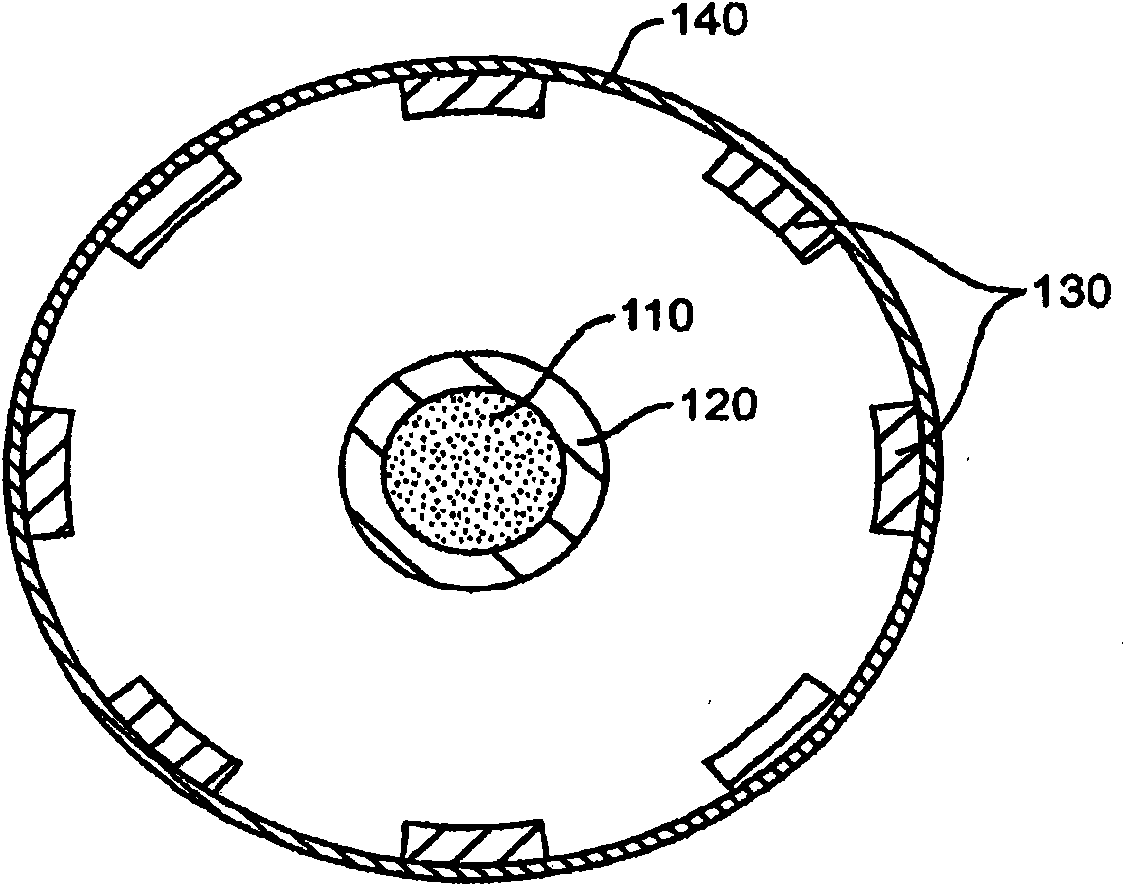
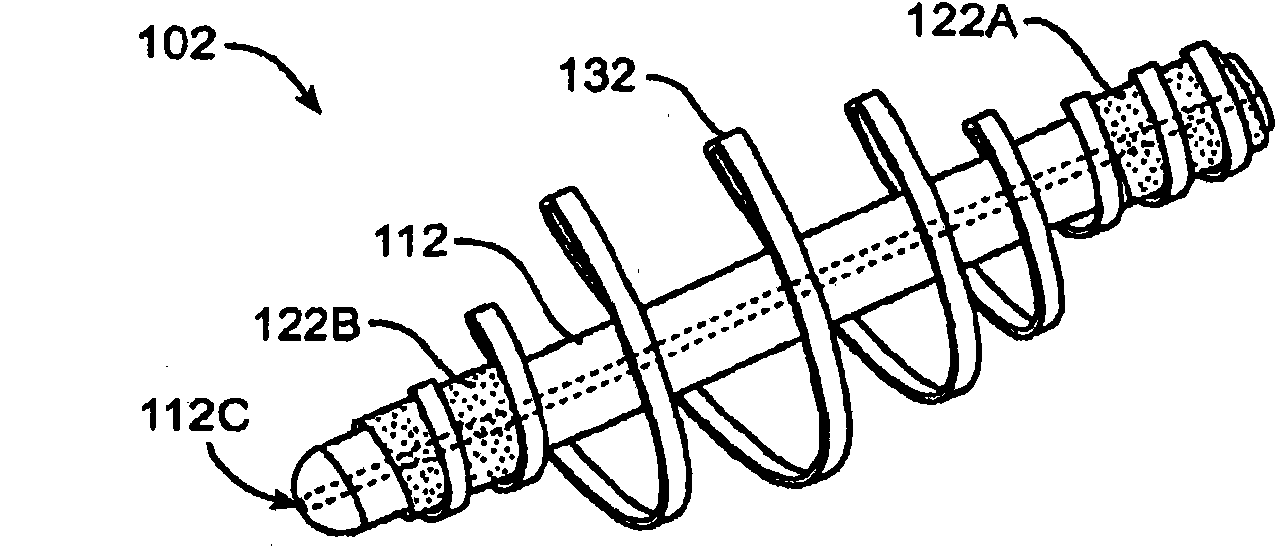
Drug cores for sustained release of therapeutic agents
A technology for the treatment of drugs and drugs, which can be used in ophthalmic treatment, drug combination, drug delivery, etc., and can solve problems such as drug shortage and continuous drug release.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0758] Latanoprost Drug Core Elution Data
[0759] Drug cores as described above have been fabricated with different cross-sectional sizes (0.006 inches, 0.012 inches and 0.025 inches) and different drug concentrations in the silicone matrix (5%, 10% and 20%). These drug cores can be produced by mixing latanoprost with silicone using a Syringe Tube and Cartridge Assembly, and injecting the mixture into a polyimide tube, which is cut into the desired length and seal. The length of the drug core is about 0.80-0.95 mm for a diameter of 0.012 inches (0.32 mm), corresponding to about 3.5 μg, 7 μg and 14 μg of total Latanoprost content.
[0760]Syringe Tubing and Cartridge Assembly 1. Polyimide tubing was obtained in three different diameters, 0.006", 0.0125", and 0.025". 2. Cut polyimide tubing of different diameters into ~15 cm lengths. 3. Insert the polyimide tubing into the syringe adapter. 4. Incorporate polyimide tubing adhesive in the luer adapter (Loctite, low viscosity...
Embodiment 2
[0773] Cyclosporin Drug Core Elution Data
[0774] Drug cores were prepared as described in Example 1 with a cyclosporine concentration of 21.2%. Figure 8 A shows an elution profile showing the elution of cyclosporin from the drug core into surfactant-free buffer solution and surfactant-containing buffer solution, according to an embodiment of the present invention. Prepare buffer solutions as described above. The surfactant containing solution consisted of 95% buffer and 5% surfactant, UP-1005 Ultra Pure Fluid from Dow Corning, Midland MI. Work in connection with embodiments of the present invention indicates that, in at least some cases, in situ elution from the eye can be simulated in vitro using surfactants, since the eye can include natural surfactants in the tear film, such as surfactant protein D. The elution profile of cyclosporine into surfactant was approximately 50-100 ng per day from 30-60 days. Empirical data from tears from patient populations (eg 10 patient...
Embodiment 3
[0776] Bimatoprost Bulk Elution Data
[0777] A bulk sample of 1% bimatoprost with a known diameter of 0.076 cm (0.76 mm) was prepared. The height of each sample was determined from the weight and known sample diameter.
[0778] Table 3. Bulk Sample Sizes
[0779] sample
wt(mg)
diameter (cm)
calculated high
degree(cm)
exposed surface area
(cm 2 )
14-2-10
1.9
0.076
0.42
0.109
14-2-11
1.5
0.076
0.33
0.088
14-2-12
1.9
0.076
0.42
0.109
[0780] The calculated height is 0.33cm-0.42cm. The exposed surface area on each end of each bulk sample is about 0.045 cm 2 , providing 0.019cm for 0.42 and 0.33cm samples respectively 3 and 0.015cm 3 volume of. The area of the exposed surface of the sample calculated from the height and diameter of the drug-free sheath was approximately 0.1 cm 2. Three formulations were evaluated: 1) Silicone 4011, 1% ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com