High gradient magnetic separation of biological material
A biological material, magnetic separation technology, applied in high gradient magnetic separation, high gradient magnetic separator, magnetic separation and other directions, can solve problems such as non-specific binding, achieve simple cost and improve the effect of purification results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
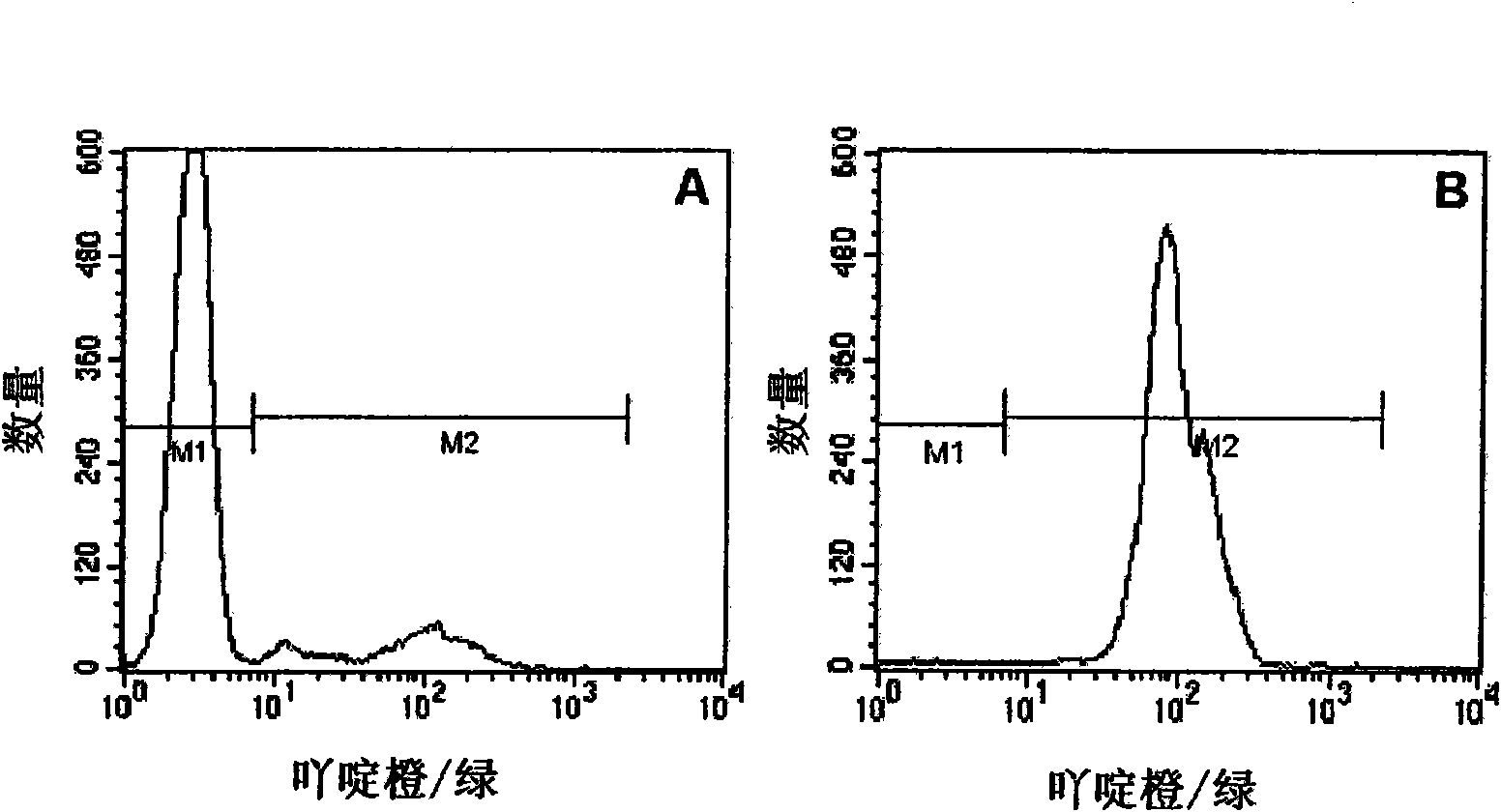
[0072] Using isotonic phosphate-buffered sucrose solution containing gelatin against Plasmodium falciparum (P.falciparum) cultures of red blood cells infected with the malaria pathogen (Plasmodium) purification.
[0073] Material:
[0074] buffer solution A 1 : Isotonic phosphate-buffered sucrose solution containing 0.75% gelatin
[0075] Stainless steel wool 1g
[0076] single tap
[0077] three way tap
[0078] 20G injection needle
[0079] 3ml disposable syringe
[0080] 10ml disposable syringe
[0081] 50ml disposable syringe
[0082] 1 neodymium horseshoe magnet
[0083] a) Preparation of purification kit
[0084] Manufacture of Separation Columns
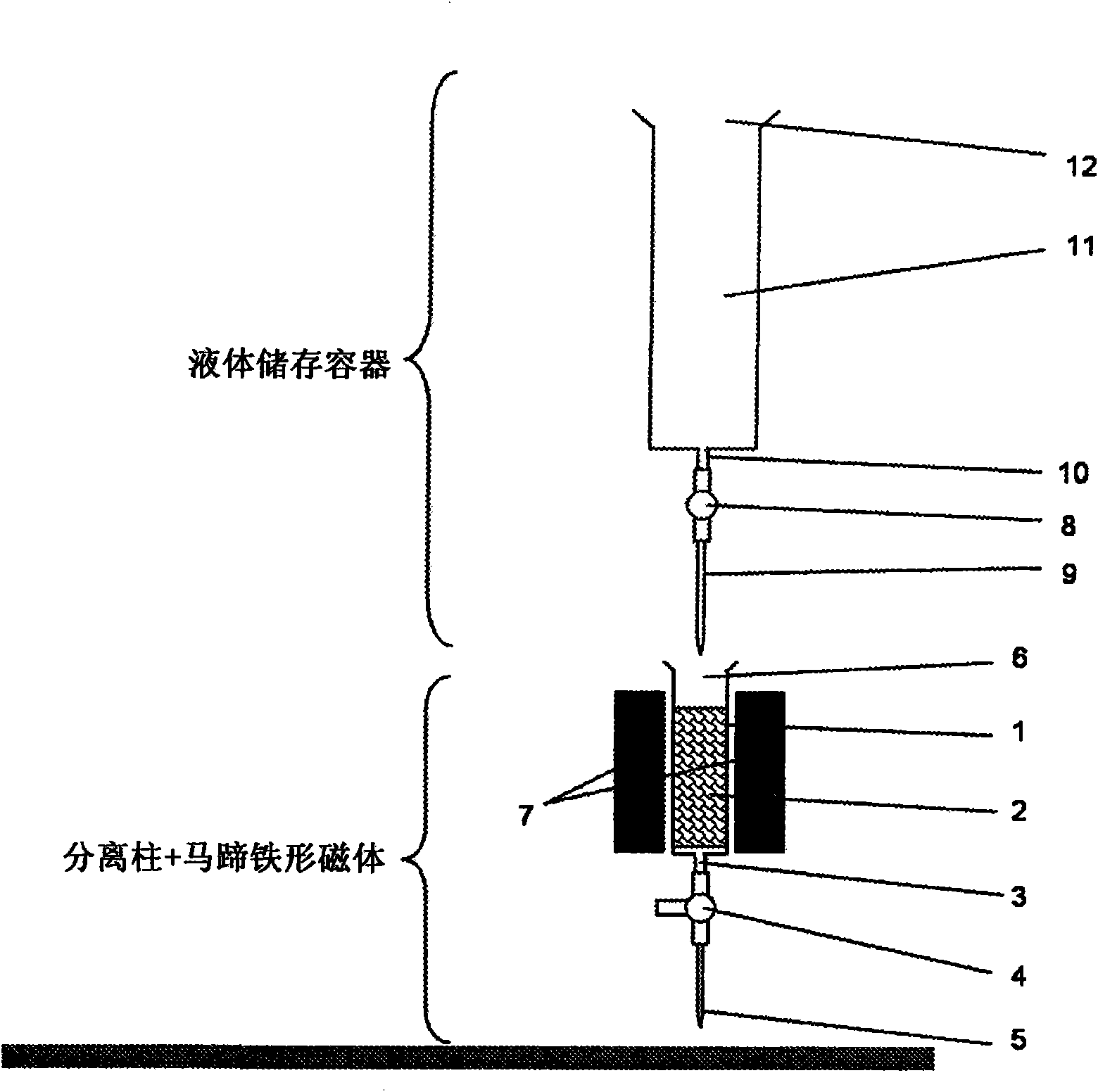
[0085] A 3 ml disposable syringe used as separation column 1 was filled with 1 gram of stainless steel wool as matrix 2 to two-thirds of the total volume of the 3 ml disposable syringe. Here, it should be noted that a large number of stainless steel wool fibers are located in the longitudinal direction of the ...
example 2
[0098] Use phosphate-buffered saline solution (PBS) containing bovine serum albumin (BSA) Purification of malaria pathogens (Plasmodium) from cultures of P. falciparum infected red blood cells
[0099] Material:
[0100] As listed in Example 1, but with Buffer A 1 buffered solution A 2 (PBS with 5% BSA) instead.
[0101] a) Preparation of purification kit
[0102] Follow the description in Example 1.
[0103] b) Preparation and execution of the separation process
[0104] Preparation of P. falciparum cultures for purification of red blood cells infected with the malarial pathogen
[0105] As in Example 1. The parasitemia of the P. falciparum culture in this experiment was 14.47%. buffer solution A 1 with buffer solution A 2 replace.
[0106] Execution of the separation process
[0107] As in Example 1, with the following differences:
[0108] buffer solution A 1 with buffer solution A 2 replace. Centrifugation of the eluate was then performed at 800 g for ...
example 3
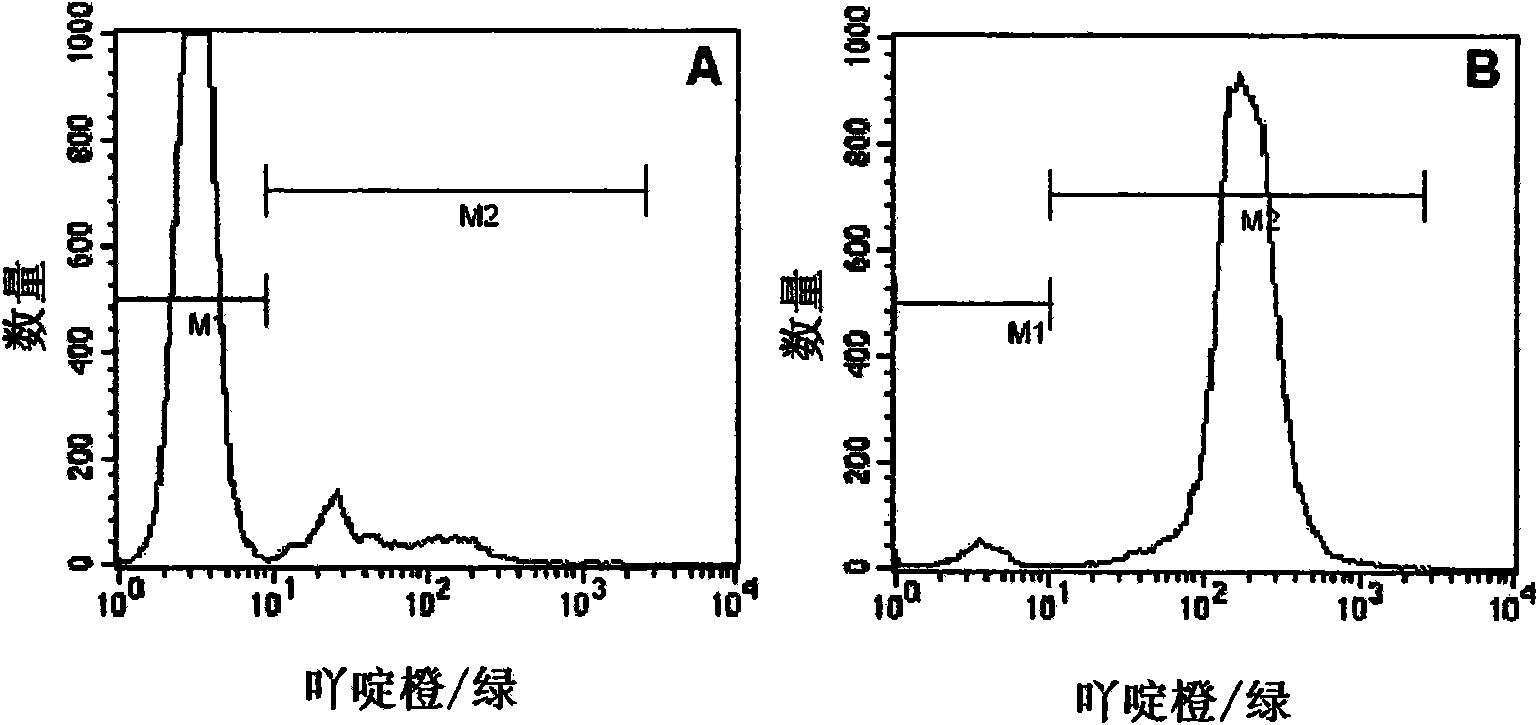
[0112] Purified from a suspension of leukocytes (peripheral blood mononuclear cells (PBMCs)) Original CD8 leukocytes (CD8 positive cells).
[0113] Material:
[0114] as listed in Example 1.
[0115] a) Preparation of purification kit
[0116] Follow the description in Example 1.
[0117] b) Preparation and execution of the separation process
[0118] Labeling of CD8-positive cells with antibody-conjugated synthetic paramagnetic particles (microbeads)
[0119] will be 1.5x10 7 Individual human peripheral mononuclear cells (PBMCs) were incubated with monoclonal rat anti-human CD8 IgG antibody in PBS / BSA 1% for 30 minutes on ice. The cells were washed twice with the same buffer solution, and then incubated with anti-rat IgG-coupled microbeads (MiltenyiBiotech GmbH, loc. cit.) for an additional 10 minutes on ice. The cells were washed twice in the same buffer solution, and then with fluorescein-labeled antibodies (PE-anti-CD8 antibody and FITC-anti-CD3 antibody, Simulte...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Gel strength | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com