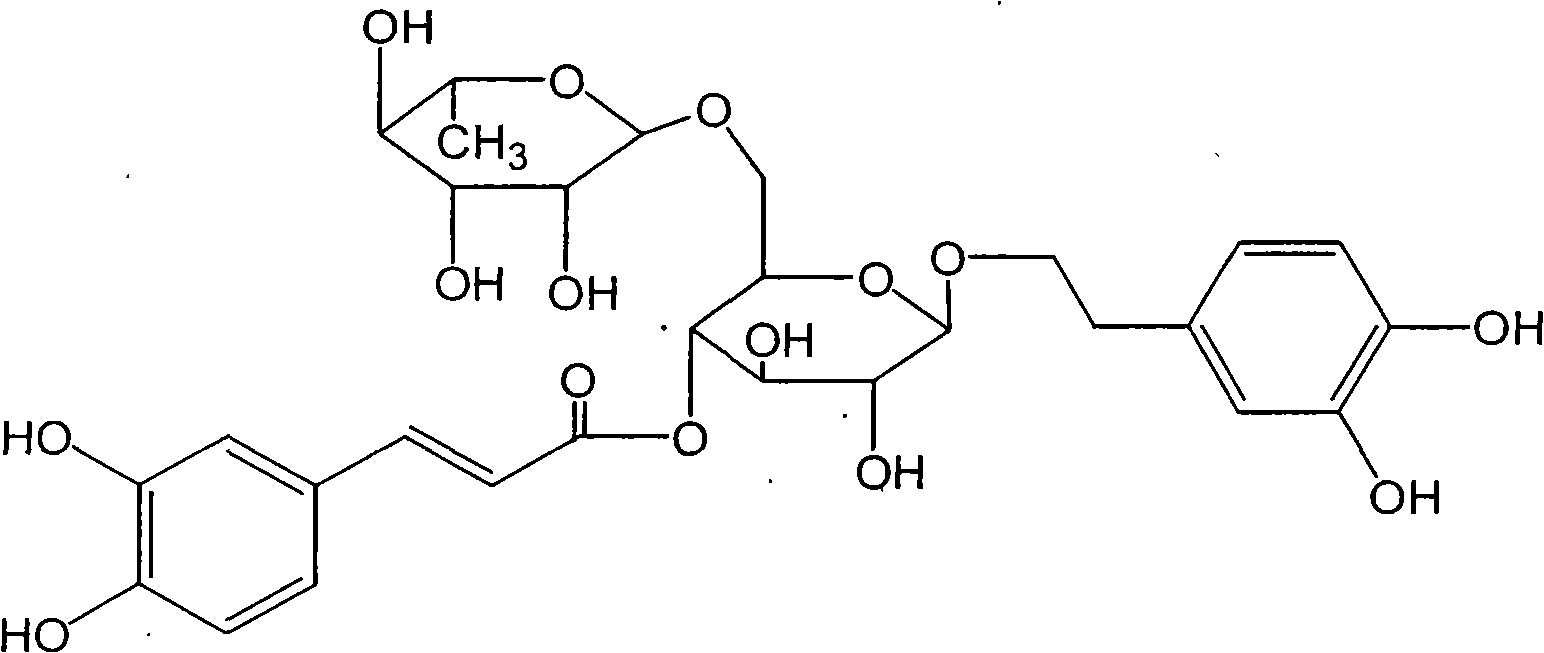
Application of orsythoside A in preparation of medicament for treating herpes simplex
A technology of forsythiaside and herpes simplex, which is applied in the field of medicine and can solve the problem of few studies on the anti-herpes virus pharmacological activity of forsythiaside
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
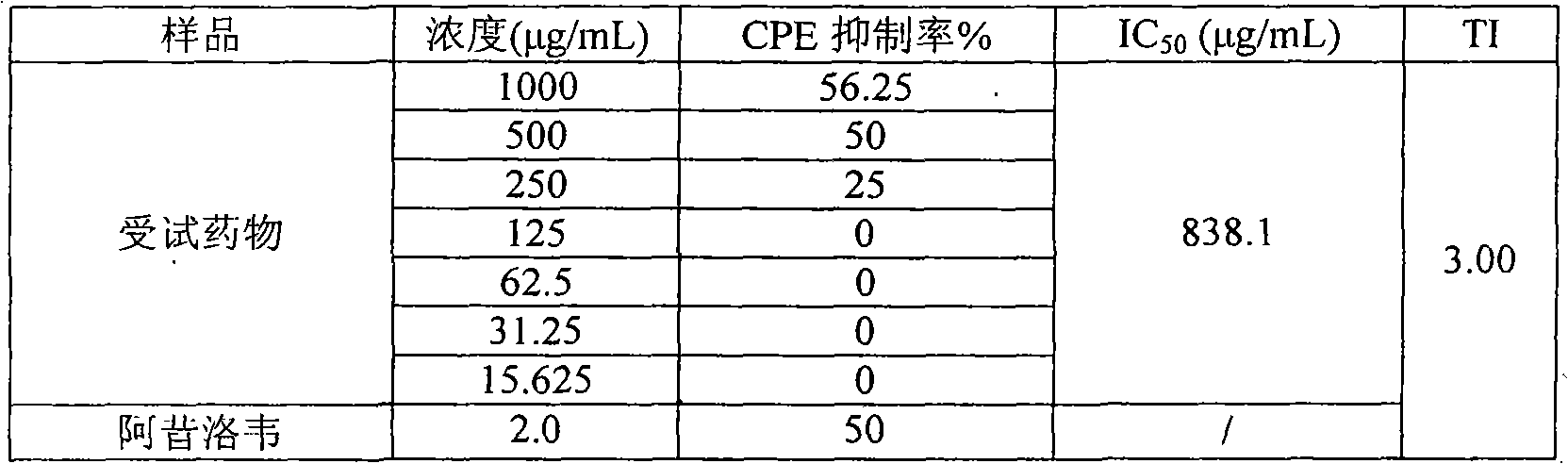
[0013] Embodiment 1 (forsythiaside anti-herpes simplex type II virus test in vitro)
[0014] 1. Experimental purpose: To observe the inhibitory effect of forsythiaside on herpes simplex type II virus in vitro.
[0015] 2. Experimental materials:
[0016] 1. Test drug: Forsythiaside, provided by Shanghai Yusen New Drug Development Co., Ltd., batch number: 060909 (For the preparation method of this drug, please refer to Chinese patents CN101081855A, CN101081856A, CN101081857A, CN1899382A, CN101209286A.)
[0017] 2. Positive control drug: Acyclovir for injection, produced by Hubei Qianjiang Pharmaceutical Co., Ltd., batch number 20070302
[0018] 3. Virus strain: herpes simplex virus (HSV) standard strain type II 333 strains, provided by the Institute of Pharmaceutical Biotechnology, Chinese Academy of Medical Sciences, Beijing.
[0019] 4. Vero cells
[0020] 3. Experimental method
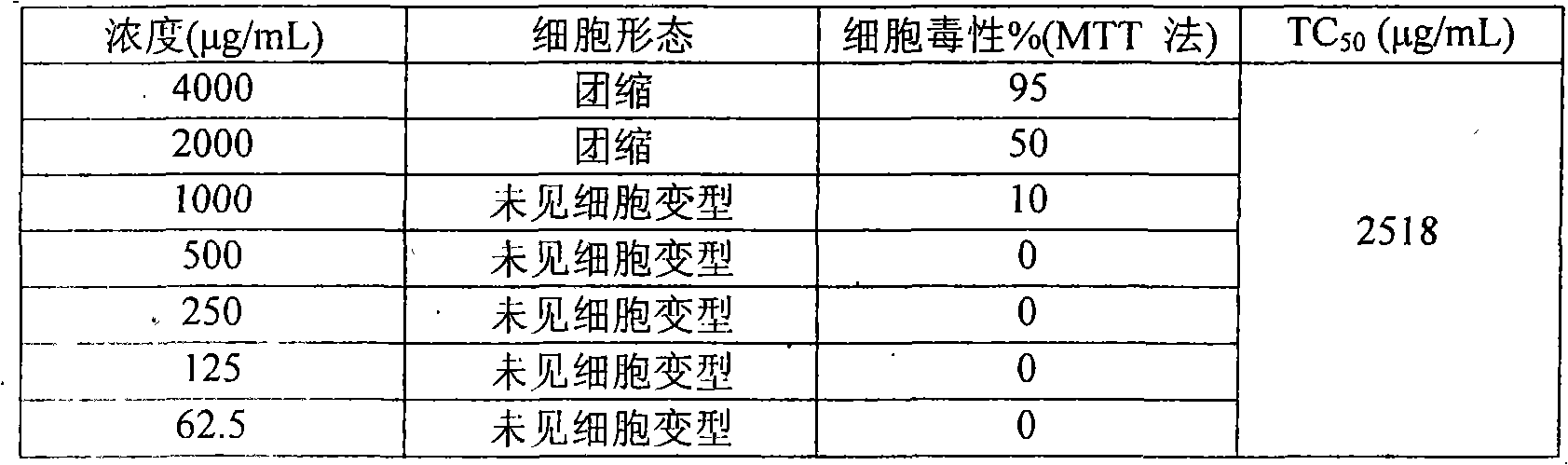
[0021] 1. Drug cytotoxicity test:
[0022] Vero cells were cultured in a 96-well culture pl...
Embodiment 2
[0035] Embodiment 2 (forsythiaside anti-herpes simplex type II virus test in vivo)
[0036] 1. Purpose of the test:
[0037] To observe the pharmacodynamic effect of forsythiaside on the inhibition of vaginitis induced by herpes simplex virus HSV-II in mice in vivo.
[0038] 2. Test materials:
[0039] Test drug: the same test drug as in Example 1.
[0040] Positive control drug: the same positive control drug as in Example 1.
[0041] Animal: Kunming mouse, weighing 17-20 grams, female. Provided by the Department of Laboratory Animal Science, Fudan University, license number: scxk Shanghai 2007-0002.
[0042] Strain: herpes simplex virus (HSV) standard strain type II 333, provided by the Institute of Pharmaceutical Biotechnology, Chinese Academy of Medical Sciences, Beijing.
[0043] Experimental drug solution preparation:
[0044] (1) The test drug for the test solution
[0045] A.24mg / cm 2 Dosage group: take forsythiaside freeze-dried powder and dissolve it in physi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com