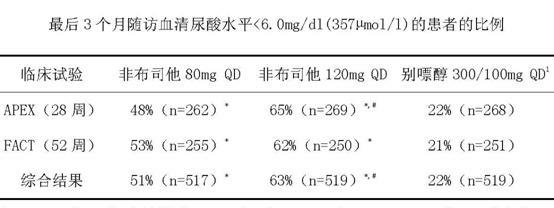
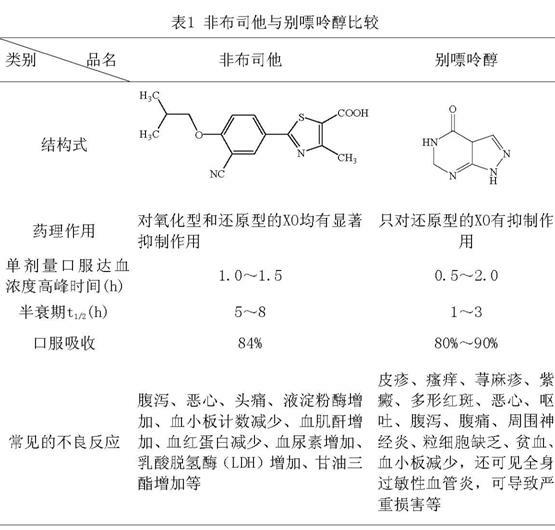
Febuxostat dispersible tablet and preparation method thereof
A febuxostat and dispersible tablet technology, which is applied in the field of medicine, can solve the problems of incomplete dissolution and low dissolution rate of ordinary tablets, and achieve the effects of stable quality, suitable for long-term storage, and rapid and complete drug dissolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] 1000 febuxostat dispersible tablets (specification 40mg) contain the following components:
[0085] Febuxostat 40g 17.1%
[0086] Microcrystalline Cellulose 120g 51.2%
[0087] Croscarmellose Sodium 60g 25.6%
[0088] Hypromellose 5g 2.1%
[0089] Silica 3g 1.3%
[0090] Magnesium Stearate 1g 0.4%
[0091] Aspartame 2.8g 1.2%
[0092] Flavor 2.5g 1.1%
[0093] Febuxostat dispersible tablets are prepared through the following steps:
[0094] The first step: pulverize febuxostat with airflow, and control the average particle size below 30 μm;
[0095] Step 2: Weigh the main ingredient febuxostat and auxiliary materials according to the prescription amount, including microcrystalline cellulose, croscarmellose sodium, hypromellose, silicon dioxide, magnesium stearate, aspirin Pass through a 100-mesh sieve for Patan and essence, and set aside;
[0096] Step 3: Mix the sieved part of the disintegrant, 57g of croscarmellose sodium (this part of the disintegrant accoun...
Embodiment 2
[0104] 1000 febuxostat dispersible tablets (specification 80mg) contain the following components:
[0105] Febuxostat 80g 18.3%
[0106] Lactose 230g 52.8%
[0107] Cross-linked polyvinylpyrrolidone 100g 23.0%
[0108] Hypromellose 8g 1.8%
[0109] Silica 6.2g 1.3%
[0110] Magnesium Stearate 2.3g 0.4%
[0111] Aspartame 6g 1.2%
[0112] Flavor 5.5g 1.1%
[0113] Febuxostat dispersible tablets are prepared through the following steps:
[0114] The first step: pulverize febuxostat with airflow, and control the average particle size below 30 μm;
[0115] Step 2: Weigh the main ingredient febuxostat and auxiliary materials according to the prescription amount, including lactose, cross-linked polyvinylpyrrolidone, hypromellose, silicon dioxide, magnesium stearate, aspartame and essence, Pass through a 100-mesh sieve and set aside;
[0116] Step 3: Mix the sieved part of the disintegrant, 50 g of cross-linked polyvinylpyrrolidone (this part of the disintegrant accounts for...
Embodiment 3
[0124] 1000 febuxostat dispersible tablets (specification 40mg) contain the following components:
[0125] Febuxostat 40g 21.7%
[0126] Lactose 90g 48.9%
[0127] Cross-linked polyvinylpyrrolidone 22g 12.0%
[0128] Low-substituted hydroxypropyl cellulose 18g 9.8%
[0129] Hypromellose 3.5g 1.9%
[0130] Silica 2.5g 1.4%
[0131] Magnesium Stearate 2g 1.1%
[0132] Aspartame 3.2g 1.7%
[0133] Flavor 2.8g 1.5%
[0134] Febuxostat dispersible tablets are prepared through the following steps:
[0135] The first step: pulverize febuxostat with airflow, and control the average particle size below 30 μm;
[0136] Step 2: Weigh the main ingredient febux and auxiliary materials according to the prescription amount, including microcrystalline cellulose, cross-linked polyvinylpyrrolidone, low-substituted hydroxypropyl cellulose, silicon dioxide, magnesium stearate, asper Tan and essence are passed through a 100-mesh sieve respectively, and set aside;
[0137] The third step:...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com