Method for removing Al impurity from KCl-LiCl lithium electrolyte
An electrolyte and impurity technology, applied in the field of metallurgy, can solve the problems of increasing the production cost of high-purity metal lithium, high distillation temperature, low distillation efficiency, etc., and achieves the effects of low cost, convenient and simple operation, and omitting the technological process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
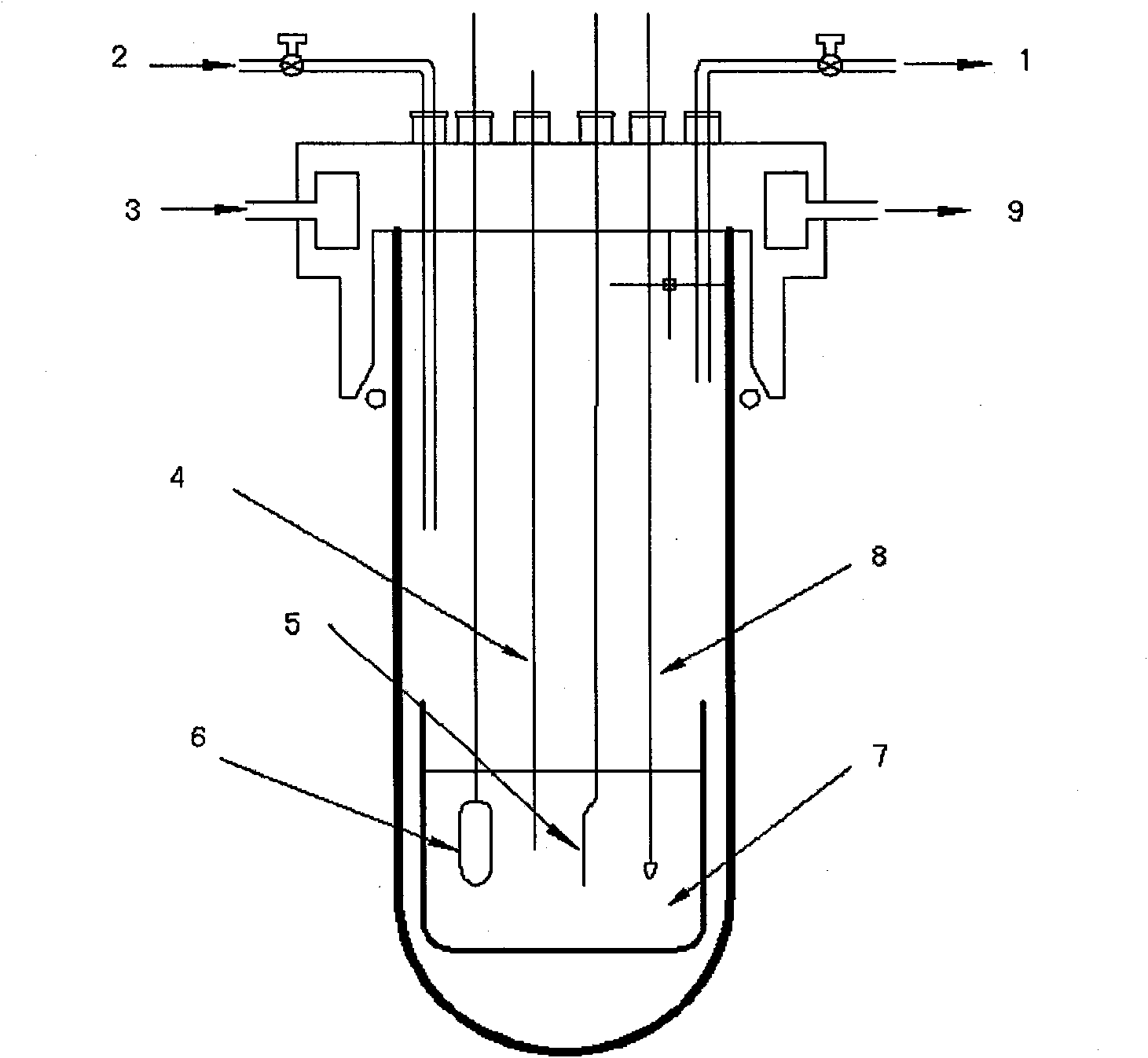
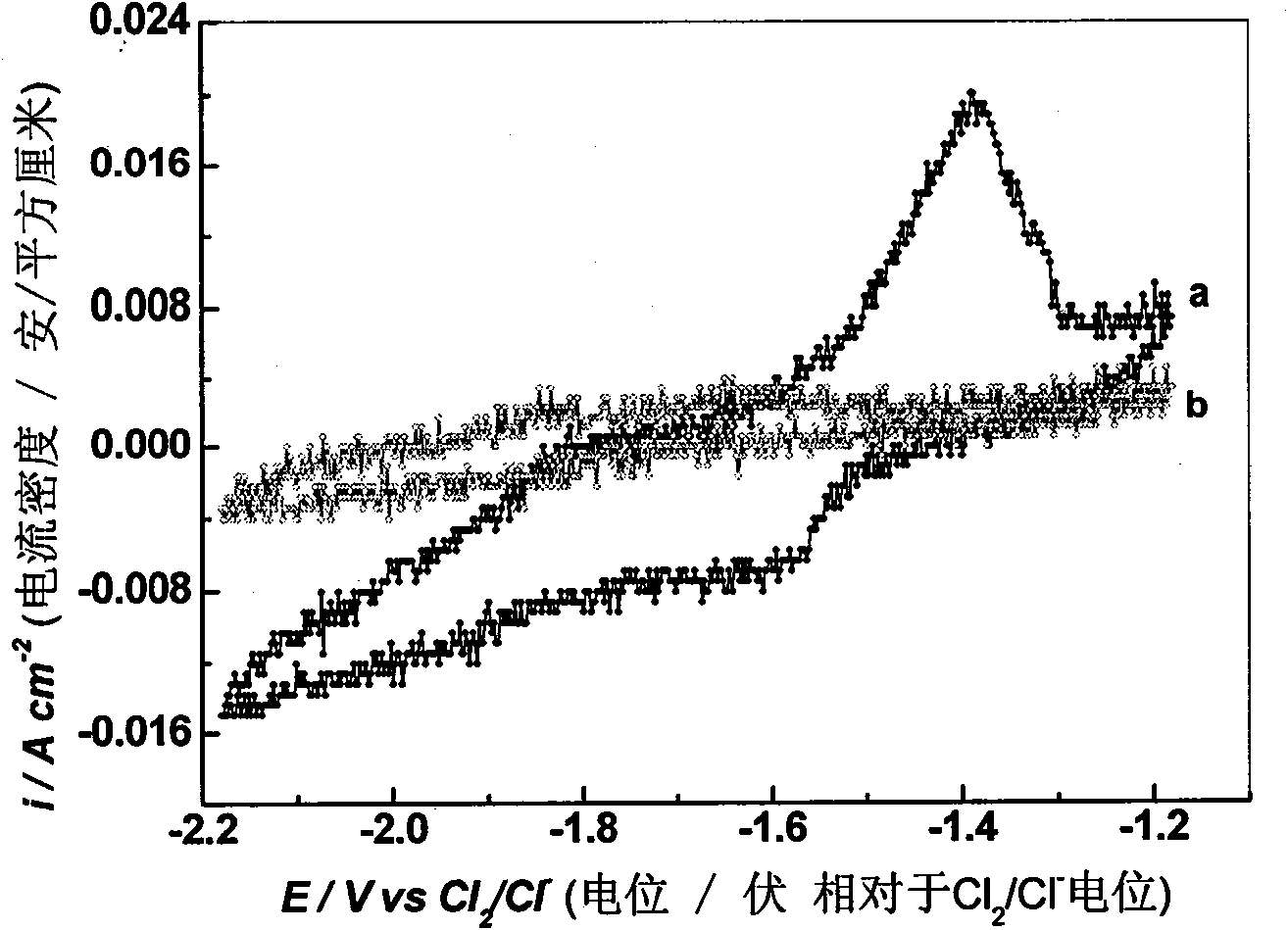
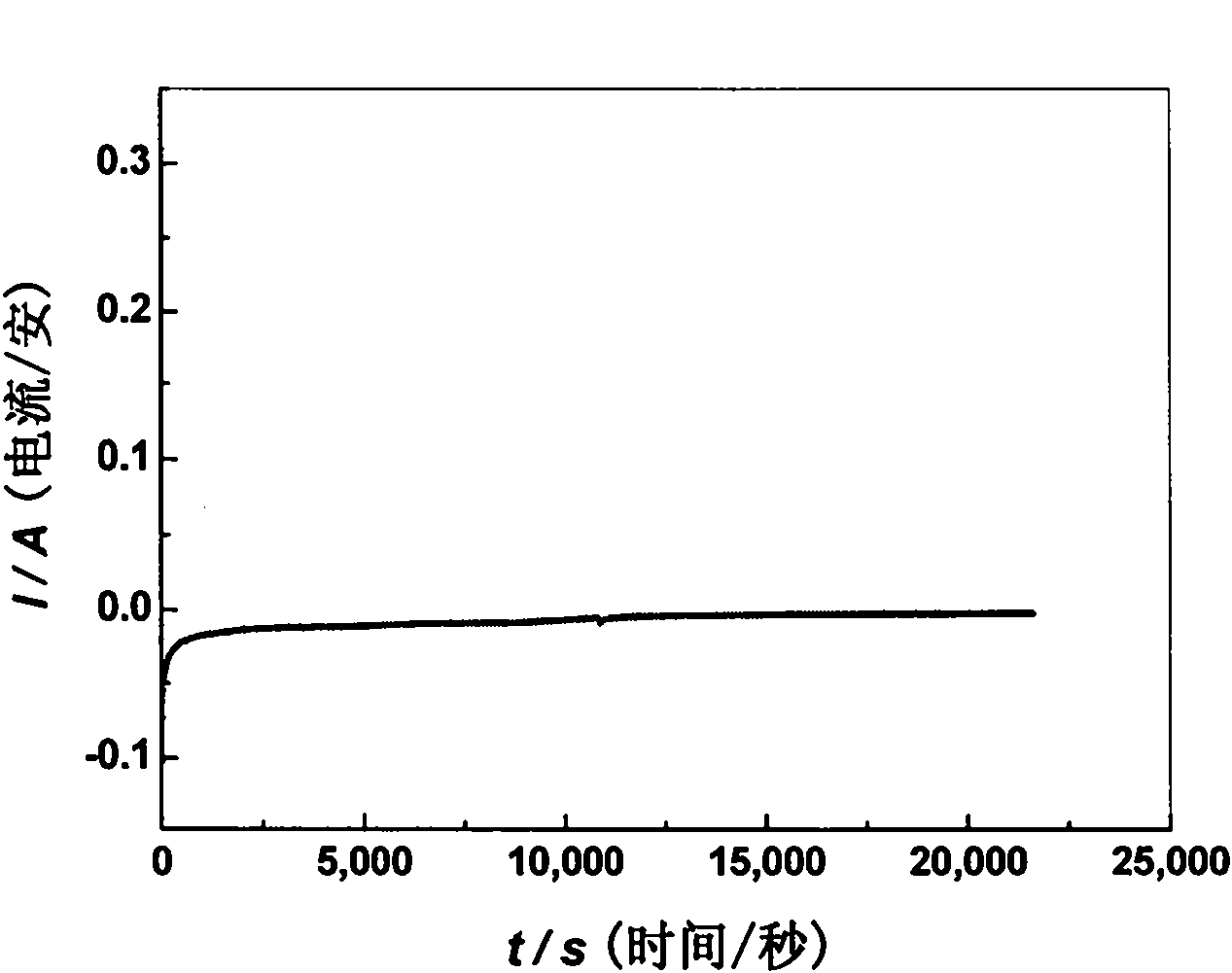
[0029] This impurity removal process uses LiCl-KCl as the basic electrolyte, adding different contents of AlCl 3 Preparation of LiCl-KCl-AlCl with different contents 3 Electrolyte system, AlCl 3 Respectively account for LiCl-KCl-AlCl 3 0.5%, 1.0%, 2.0% of the total mass; in LiCl-KCl-AlCl 3 (0.5wt%), LiCl-KCl-AlCl 3 (1.0wt%), LiCl-KCl-AlCl 3 (2.0wt%) system, adopt electrochemical method to determine the precipitation potential of Al;
[0030] The cathode carbon steel for impurity removal adopts a sheet structure of 5×4cm, graphite is used as the anode, and the cathode carbon steel is not put into the reactor before electrolysis. First use cyclic voltammetry, with W (0.1649cm 2) is the working electrode, Ag wire (inserted into a ceramic tube equipped with LiCl-KCl-AgCl electrolyte, wherein the molar ratio of LiCl: KCl is 1:1, and the molar number of AgCl is 4% of the total molar number of LiCl-KCl-AgCl) As a reference electrode, spectroscopically pure graphite is used as ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com