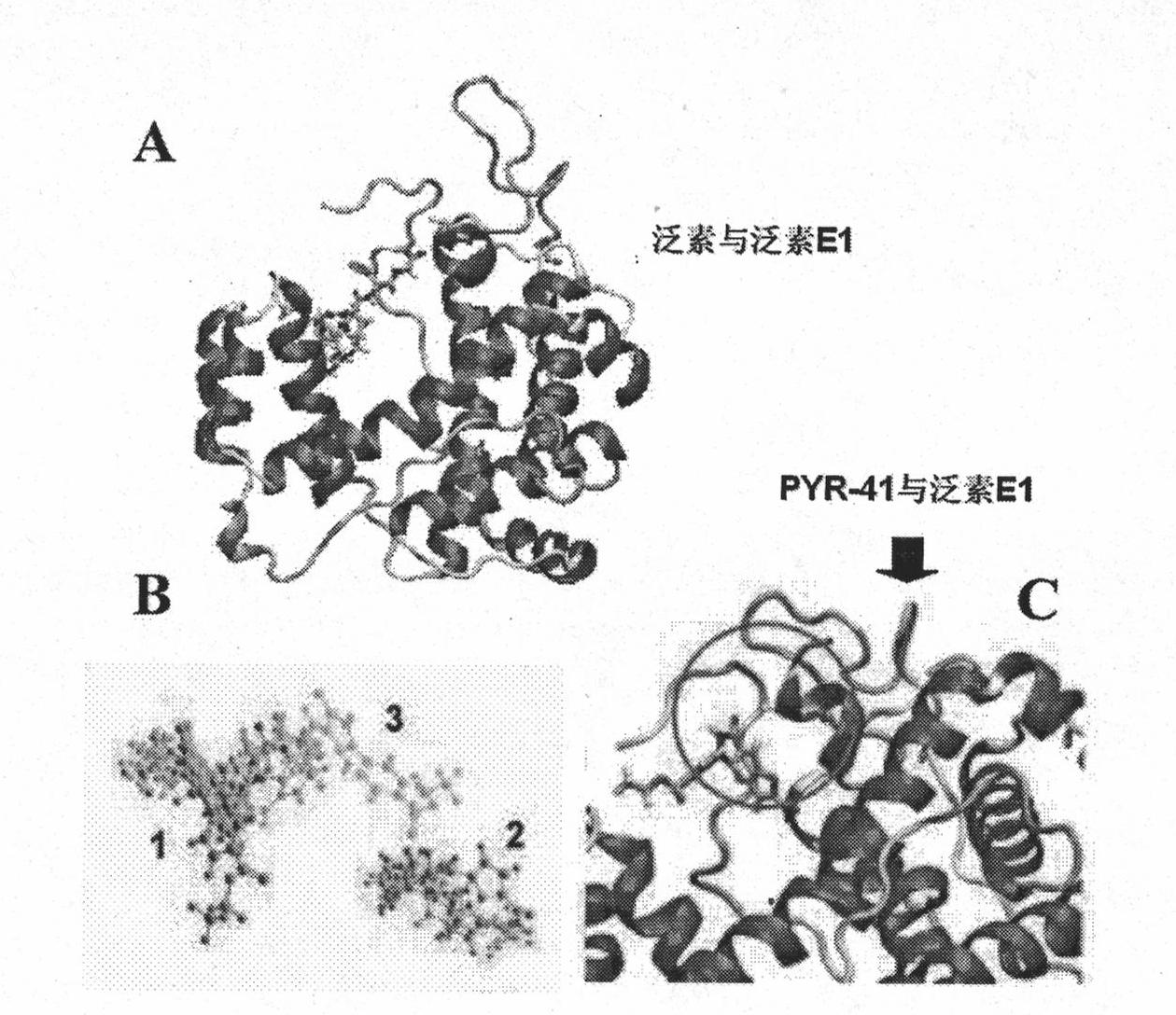
Ubiquitin E1 inhibitor and preparation method thereof
A technology of inhibitors and ubiquitin, applied in the field of compounds of ubiquitin E1 inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086] Example 1: Preparation of ubiquitin E1 inhibitor compounds and derivatives thereof
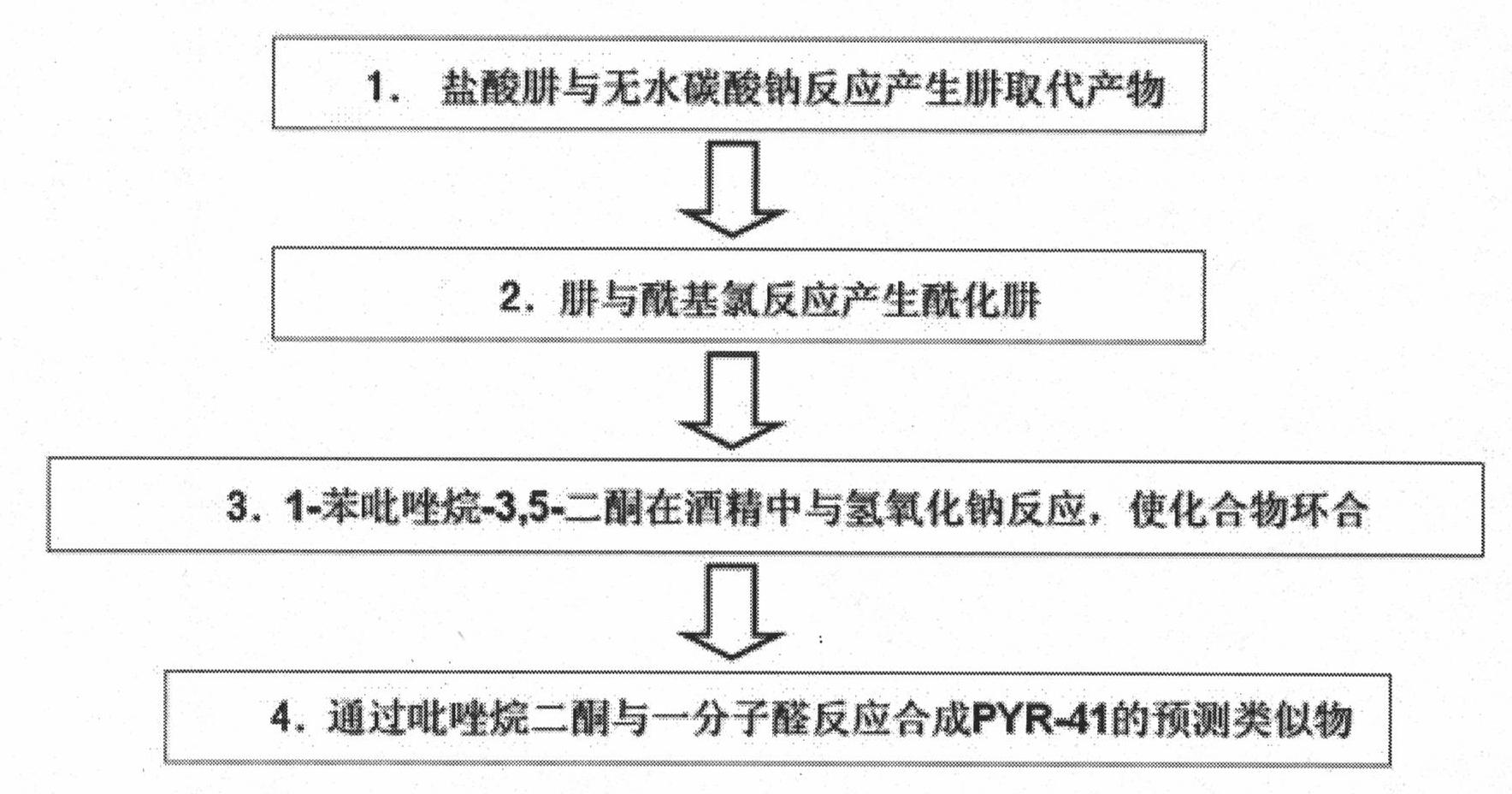
[0087] figure 2 Show the preparation method and technological process of present embodiment:
[0088] (a) prepare alkali-free hydrazine group, take phenylhydrazine as example (see scheme II):
[0089] Anhydrous sodium carbonate (0.015M) was added to 50ml of an aqueous solution containing 0.01M phenylhydrazine hydrochloride, and extracted three times with dichloromethane, 30ml each time, to obtain a solution. It was dried over anhydrous sodium carbonate and concentrated to release phenylhydrazine, which was used in subsequent synthesis without purification.
[0090] (b) Preparation of compound (iii), methyl-3-oxo-3-(N'-phenylhydrazino) propionic acid
[0091] Dissolve phenylhydrazine (5.94 g, 55 mM) in 100 ml dry THF and Et 3 H (8.11 ml, 58.3 mM). The mixed reaction was cooled to -10°C, and 50 ml of anhydrous THF containing methyl-3-chloro-3-oxo-propionic acid (7.12 ml, 56.6 mM) wa...
Embodiment 2
[0096] Example 2: Detection of ubiquitin E1 inhibitory effect of ubiquitin E1 inhibitor compounds and derivatives thereof
[0097]Experimental method: use DMEM / F-12 medium (Hyclone, logan, UT) (wherein containing 10% FCS, 0.3% sodium bicarbonate, 100U / ml penicillin, 100 μ g / ml streptomycin) to cultivate RPE cells for detection Ubiquitin E1 inhibitors penetrate into cells and bind intracellularly to the level of E1 ubiquitin complexes. The cells were seeded in a 12-well tissue culture plate and cultured overnight, and the cell confluency reached 85%. Incubate with DMSO, PYR-41 (50 μM) and compound (50 μM) for 30 minutes. Wash gently twice with PBS, add lysis buffer to scrape off the cells (lysis buffer: 5mM Tris-HCl, pH6.8, 8.7M urea, 1% NP-40, 20mM N-hexylmaleimide , 3mM EDTA, 10mM iodoacetamide and mixed protease inhibitors), simple sonication, 12000 rpm, centrifugation at 4°C for 20 minutes, and the supernatant was collected. Divide the sample into 2 equal parts, add to 4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com