3,4,6-triaryl-(1,3)-oxazine-2-ketone compound as well as preparation method and application thereof
A technology of ketone compounds and compounds, applied in the field of non-steroidal drugs, can solve problems such as rising, tumor volume increase, blurring, etc.
Inactive Publication Date: 2011-01-19
SICHUAN UNIV
View PDF3 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
However, it has been found in clinical use that both steroidal and non-steroidal AR antagonists have certain side effects, including estrogen and androgen metabolic disorders, gynecomastia and liver toxicity; Side effects such as gastrointestinal discomfort, nausea, vomiting, insomnia, fatigue, headache, anxiety, blurred vision, and loss of libido may occur with hetamine or bicalutamide; secondly, patients with benign prostatic hyperplasia / prostate cancer take a single antiandrogen drug Later, "Antiandrogen Withdrawal Syndrome" (AWS) will appear, which is manifested by the rapid increase of the suppressed prostate-specific antigen (Prostate-specific Antigen, PSA) level and tumor volume after a period of medication , can only stop taking the drug or switch to other anti-androgen drugs
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
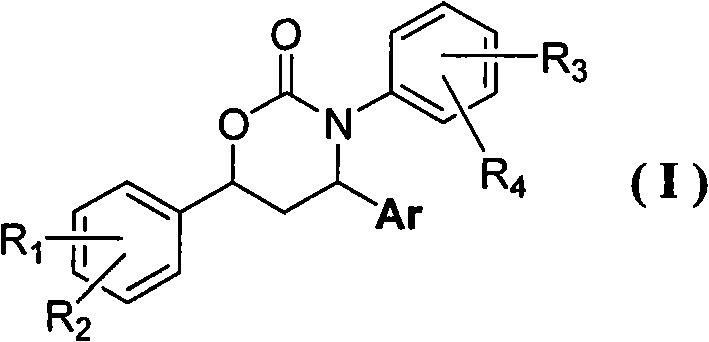
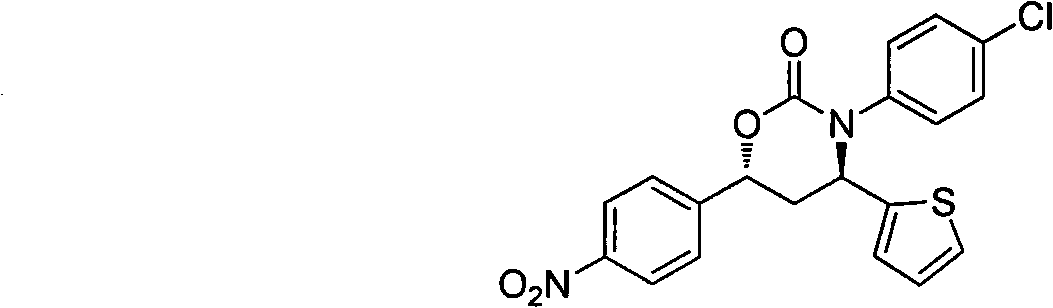
The invention belongs to the field of pharmaceutical chemistry and relates to a 3,4,6-triaryl-(1,3)-oxazine-2-ketone compound, a preparation method thereof and also a drug composition containing the compound in a formula (I). The 3,4,6-triaryl-(1,3)-oxazine-2-ketone compound and the drug composition are used as an androgen receptor antagonist and can be used for preparing a non-steroidal drug for preventing and / or treating prostatic hyperplasia, prostate cancers, hirsutism, severe androgen-dependent alopecia or acne symptoms or diseases, associated with androgen receptors.
Description
Technical field The invention belongs to the field of medicinal chemistry, and relates to 3,4,6-triaryl-[1,3]-oxazin-2-one compound (I) and its preparation method, pharmaceutical composition and preparation for prevention or / and treatment Androgen receptor-related prostate hyperplasia, prostate cancer, women's hirsutism, severe androgen-dependent alopecia or acne symptoms or diseases in non-steroidal drugs. Where R 1 , R 2 , R 3 , R 4 Represents H, halogen, C 1 ~C 12 Alkyl, C 1 ~C 12 Alkoxy, nitro, cyano, carboxyl, hydroxyl, CF 3 , NR 6 R 7 ; R 6 , R 7 Represents H, C 1 ~C 12 Alkyl, C 6 ~C 12 Aryl; R 1 , R 2 , R 3 , R 4 Can be in any possible position of the benzene ring, R 1 , R 2 , R 3 , R 4 They may be the same or different; Ar represents an aryl group or a substituted aryl group. Background technique Androgen (Androgen) is a general term for hormones such as testosterone, dihydrotestosterone, and adrenal androsterone. It is mainly secreted by the interstitial cells of the ...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C07D413/04C07D265/10A61K31/5355A61K31/535A61P5/28A61P13/08A61P35/00A61P17/14A61P17/10A61P19/10A61P21/00A61P15/00A61P1/14A61P25/28A61P25/16
Inventor 邓勇黄志雄周黎丽
Owner SICHUAN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com