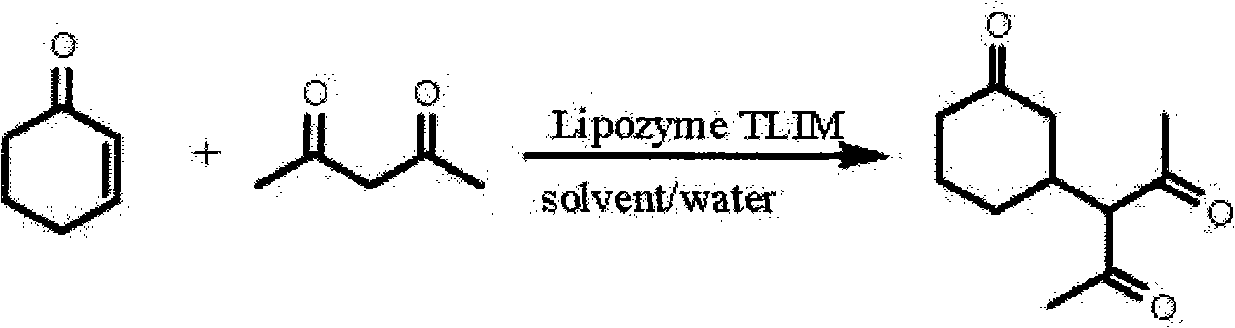Application of immobilized lipase Lipozyme TLIM as catalyst for unsymmetrical Michael addition reaction
A technology of immobilizing lipase and addition reaction, applied in the direction of fermentation, etc., to achieve the effect of good selectivity, good catalytic activity and selectivity, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~11
[0014] Examples 1-11, Michael addition reactions catalyzed by different enzymes
[0015]
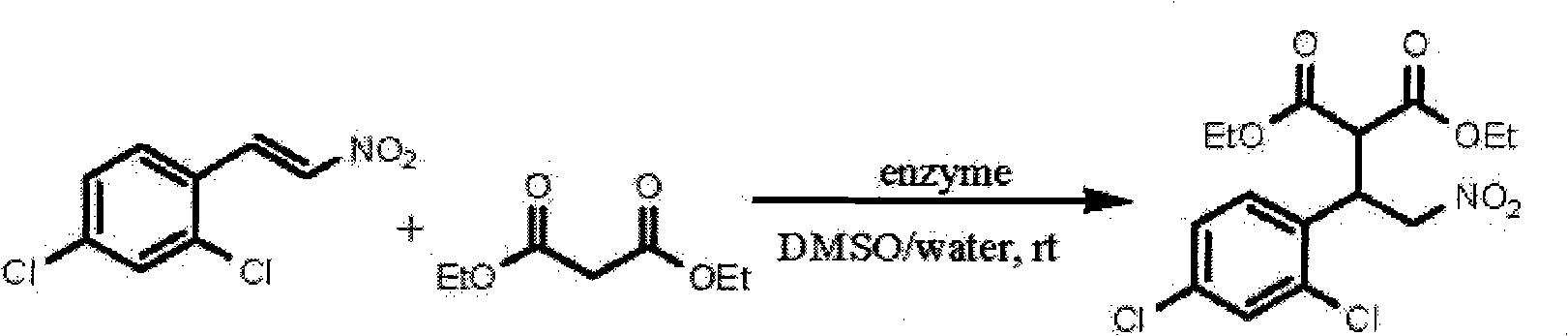
[0016] In a round bottom flask was added trans-2,4-dichloro-β-nitrostyrene (120 mg, 0.55 mmol), diethyl malonate (177 mg, 1.1 mmol), deionized water (1 mL), dimethyl Sulfoxide (DMSO, 5mL) and enzyme (400mg) were stirred and reacted at room temperature (25-30°C); after the reaction was completed, filtered, the filter cake was washed with dichloromethane, the washing liquid was combined with the filtrate, diluted with water, and then used Extract with dichloromethane, combine the dichloromethane extracts, dry with anhydrous sodium sulfate, distill off the solvent under reduced pressure to obtain a crude product, and purify the crude product by flash column chromatography to obtain the target product. The reaction conditions and results are shown in Table 1.
[0017] Table 1 Michael addition reactions catalyzed by different enzymes
[0018]
[0019] As can be seen from Table 1, the ...
Embodiment 12~46
[0020] Examples 12-46, Lipozyme TLIM catalyzes the asymmetric Michael addition reaction of 2-cyclohexenone and acetylacetone
[0021]
[0022] Add 2-cyclohexenone, acetylacetone, deionized water, organic solvent and Lipozyme TLIM in a round-bottomed flask, and stir the reaction at a certain temperature; Combine with the filtrate, dilute with water, extract with dichloromethane, combine the dichloromethane extracts, dry with anhydrous sodium sulfate, distill off the solvent under reduced pressure to obtain a crude product, purify the crude product with flash column chromatography to obtain target product. The reaction conditions and results are shown in Table 2.
[0023] Table 2. Asymmetric Michael addition reaction of 2-cyclohexenone and acetylacetone catalyzed by Lipozyme TLIM
[0024]
[0025]
[0026] Note: The ee value was not detected in Examples 35-46.
[0027] It can be seen from Table 2 that the asymmetric Michael addition reaction catalyzed by the immobili...
Embodiment 47~51
[0028] Examples 47-51, Asymmetric Michael addition reaction of 4-chloro-β-nitrostyrene and ethyl acetoacetate
[0029]
[0030] Add 4-chloro-β-nitrostyrene (3.0mmol), ethyl acetoacetate (1.0mmol), deionized water (0.5mL), DMSO (5mL) and catalyst (200mg) in a round bottom flask, 35°C Stir the reaction; after the reaction is completed, filter, wash the filter cake with dichloromethane, combine the lotion with the filtrate, dilute with water, extract with dichloromethane, combine the dichloromethane extracts, dry with anhydrous sodium sulfate, reduce The solvent was distilled off under high pressure to obtain a crude product, which was purified by flash column chromatography to obtain the target product. The reaction conditions and results are shown in Table 3.
[0031] Table 3. Asymmetric Michael addition reaction of 4-chloro-β-nitrostyrene with ethyl acetoacetate
[0032]
[0033] It can be seen from Table 3 that the Michael addition reaction of 4-chloro-β-nitrostyrene ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com