4,4'-dimercaprol azobenzene and preparation method thereof
A technology of dimercaptoazo and diiodoazobenzene, applied in 4 fields, can solve the problems of poor reaction selectivity, high toxicity of reagents, low yield and the like, and achieve the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] (1) Preparation of 4-iodoaniline (2)
[0042] Add NaHCO to a 1L round bottom flask 3 (15.16g, 180mmol) and water (250mL), after completely dissolving, add aniline (11.18g, 10.94mL, 120mmol), add ice cubes to cool the solution to 12-15°C, add I in batches within 15min under vigorous stirring 2 (25.38g, 100mmol), suction filtration after 30min reaction, the dark gray solid crude product can be obtained by saturated NaHSO 3 Decolorization, sublimation at 40 ° C after drying in the air to give 4-iodoaniline (2).
[0043] Yield: 16.4 g, 75%.
[0044] Physical properties: white needle-like crystals; melting point (M.p.) is 66°C; molecular formula I-C 6 h 4 -NH 2 ; Molecular weight 219.02; Soluble in any organic solvent.
[0045] Spectroscopy data:
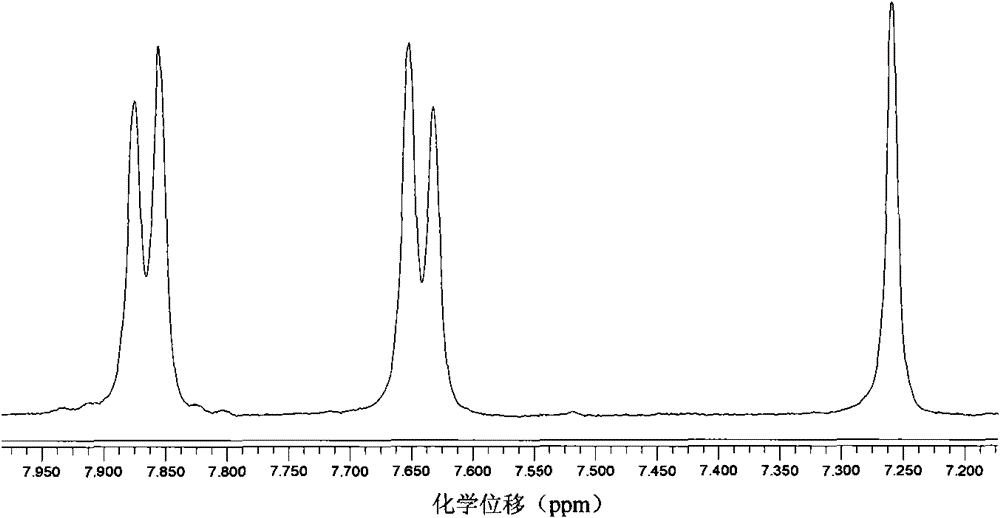
[0046] 1 H NMR (400MHz, CDCl 3 , 298K, ppm): δ7.39(d, 3 J H,H =8.8Hz, 2H), 6.46(d, 3 J H,H =8.8Hz, 2H), 3.477(s, 2H).
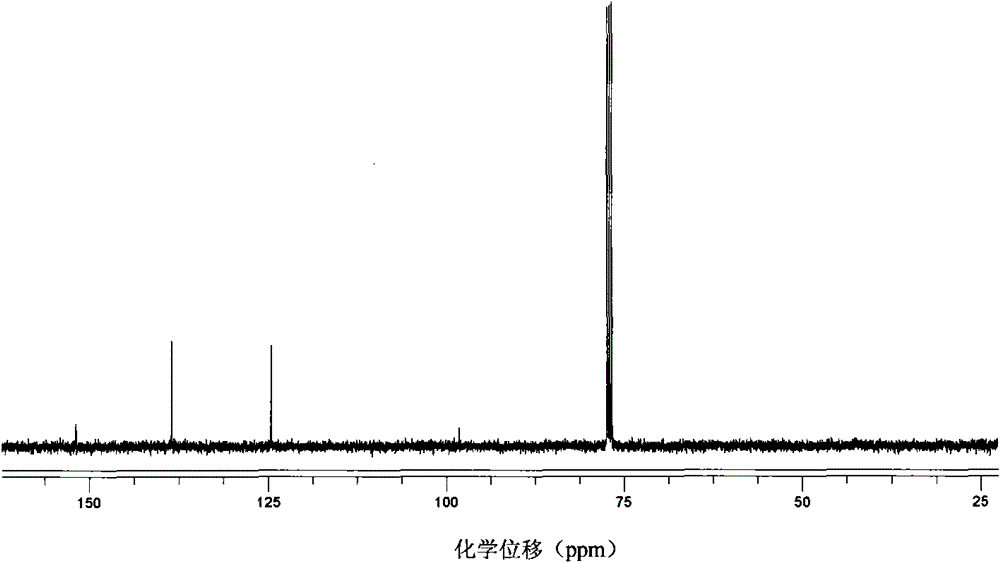
[0047] 13 C NMR (100MHz, CDCl 3 , 298K, ppm): δ147.1, 138.2, 115.9, 80.1.
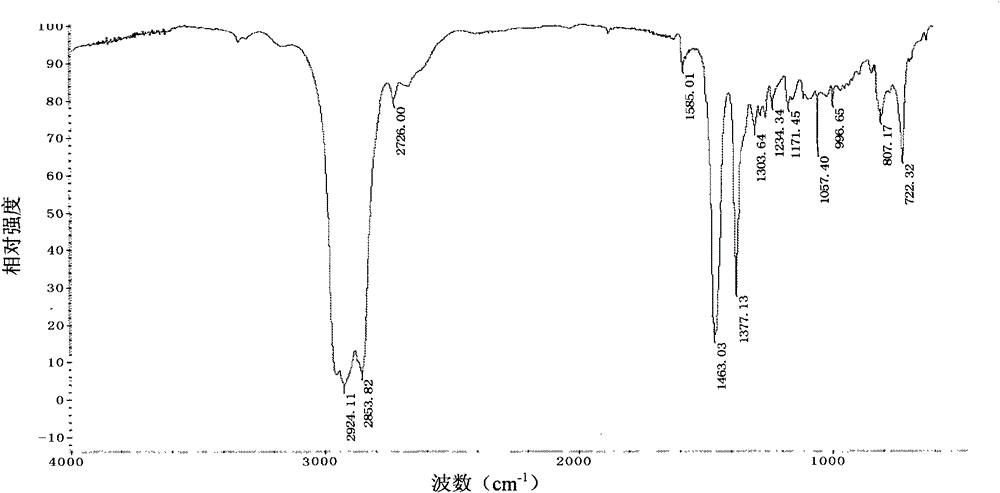
[0048] IR (KBr, c...
Embodiment 2
[0074] Similar to Example 1, the difference is that the solvent in the preparation of 4-iodoaniline (2) is dioxane and pyridine. The specific steps are: in a 1L round-bottomed flask, add aniline (9.31g, 9.12mL, 100mmol), dioxane (150mL) and pyridine (100mL), cooled to about 0°C in an ice bath, and quickly added I 2 (50.76g, 200mmol), the solution gradually turned reddish brown. After 1h, remove the ice bath, continue to stir at room temperature for 3h, add Na 2 S 2 o 3 Saturate the solution until the solution is brownish red and clear, then use CH 2 Cl 2 (150mL×2) extraction, the combined organic layers were washed with anhydrous Na 2 SO 4 After drying, the solvent was drained to a dark red solid, which was sublimed at 40°C to obtain 4-iodoaniline (2) (14.85 g, 68%) in the form of white needles.
Embodiment 3
[0076] Similar to Example 1, the difference is that KMnO is used for oxidative coupling 4 、CuSO 4 ·5H 2 O and Al 2 o 3 Supported oxidation system, the specific steps are: 6.321g (40mmol) KMnO 4 Dissolve in water to form a concentrated solution, add 15g of 200-300 mesh Al 2 o 3 Put the powder in a 250mL round-bottomed flask, stir well to form a slurry. Then weigh 9.98g (40mmol) CuSO 4 ·5H 2 O, fully pulverized with a mortar and added to the above slurry, stirred evenly, added 2.19g (10mmol) 4-iodoaniline (2) at one time, mechanically stirred for 24h, followed by TLC until the reaction was complete. The obtained purple-black solid was treated with 10 mL×3 CH 2 Cl 2 Extract and filter to obtain an orange-red solution. The obtained orange-red solution was heated to evaporate part of the solvent and then cooled to obtain orange-red flaky crystal 4,4'-diiodoazobenzene (3) (0.98g, 45%).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com