Hydrochloric acid melphalan injection liquid and preparation method thereof
A technology of melphalan hydrochloride and injection, which is applied in the direction of medical formulas, medical preparations with no active ingredients, medical preparations containing active ingredients, etc. It can solve the problems of poor long-term storage stability, time-consuming freeze-dried powder injection, and high cost. increase and other problems, to overcome the poor stability, reduce the cost of medication, and overcome the effects of energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
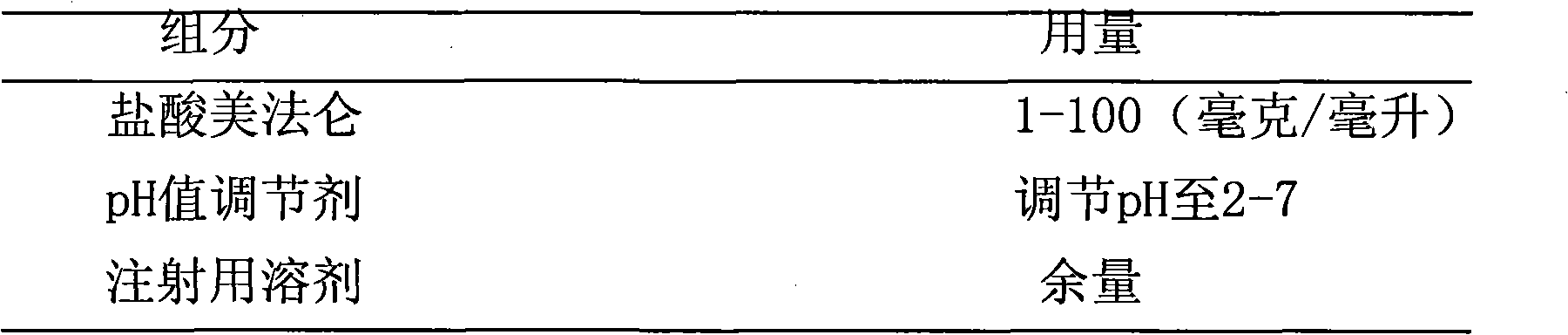
[0027] Embodiment 1 Melphalan Hydrochloride Injection
[0028] Weigh 4 grams of anhydrous sodium sulfate into 200 ml of absolute ethanol, stir and dehydrate for 15 minutes, filter to obtain dehydrated absolute ethanol; weigh 1.0 g of melphalan hydrochloride, add it to 95 ml of dehydrated absolute ethanol, and Heat and stir at 25°C to dissolve, then dilute to 100 ml with dehydrated absolute ethanol; adjust the pH value to 4.5 with citric acid; add 0.5 g of activated carbon for needles, and absorb at 45°C for 30 minutes; filter with a microporous membrane, Subpackage, seal, and sterilize with high-pressure steam at 121°C for 15 minutes to obtain melphalan hydrochloride injection.
Embodiment 2
[0029] Embodiment 2 melphalan hydrochloride injection
[0030] Weigh 30 grams of anhydrous sodium sulfate into 200 ml of propylene glycol, stir and dehydrate for 10 minutes, filter to obtain dehydrated propylene glycol; weigh 2.5 grams of melphalan hydrochloride, put it into 90 ml of dehydrated propylene glycol, heat and stir at 80°C to dissolve , and then use dehydrated propylene glycol to make the volume to 100 ml; adjust the pH value to 6.3 with hydrochloric acid; add 2 grams of activated carbon for needles, and absorb for 20 minutes at 60°C; Sterilize for 15 minutes to obtain melphalan hydrochloride injection.
Embodiment 3
[0031] Embodiment 3 melphalan hydrochloride injection
[0032] Weigh 20 grams of anhydrous calcium chloride into 200 ml of polyethylene glycol 400, stir and dehydrate for 60 minutes, and filter to obtain dehydrated polyethylene glycol 400; weigh 0.5 grams of melphalan hydrochloride into 95 ml of dehydrated polyethylene glycol 400 In ethylene glycol 400, heat and stir at 70°C to dissolve, then dilute to 100 ml with dehydrated polyethylene glycol 400; adjust the pH value to 3.5 with lactic acid; add 1.2 g of activated carbon for needles, and absorb at 80°C for 15 minutes ; filter with a microporous membrane, subpackage, seal, and sterilize with high-pressure steam at 115° C. for 30 minutes to obtain melphalan hydrochloride injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com