Peptide ligand directed drug delivery
A liposome and compound technology, applied in the field of peptide ligand-directed drug delivery, can solve the problem of weakening curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Egg Yolk Phosphatidylcholine, 1,2-Distearoyl-sn-Glyceryl-3-Phosphoethanolamine (DSPE), 1,2-Distearoyl-sn-Glyceryl-3-Phosphoethanolamine-N-[ Methoxy(polyethylene glycol)-2000] (DSPE-PEG2000), 1,2-distearoyl-sn-glyceryl-3-phosphoethanolamine-N-[maleimide (polyethylene glycol )2000] (DSPE-PEG2000-Mal) and cholesterol were purchased from Avanti Polar Lipids (AL, USA). Lissamine TM Rhodamine B, 1,2-Dihexadecanoyl-sn-Glyceryl-3-Phosphoethanolamine (Rhodamine DHPE) and N-(Fluorescein-thiocarbamoyl)-1,2-Dihexadecanoyl- sn-glyceryl-3-phosphoethanolamine (fluorescein DHPE) was purchased from Invitrogen. N-Succinimide 3-(2-pyridinedithio)-propionate (SPDP) and tris(carboxyethyl)phosphine (TCEP) were purchased from Pierce Biotechnology, Inc. Cy5.5 Mono NHS Ester was provided by GE Healthcare. All other chemicals of analytical grade were obtained from Sinopharm Chemical Reagent Co. Ltd (Shanghai, China).
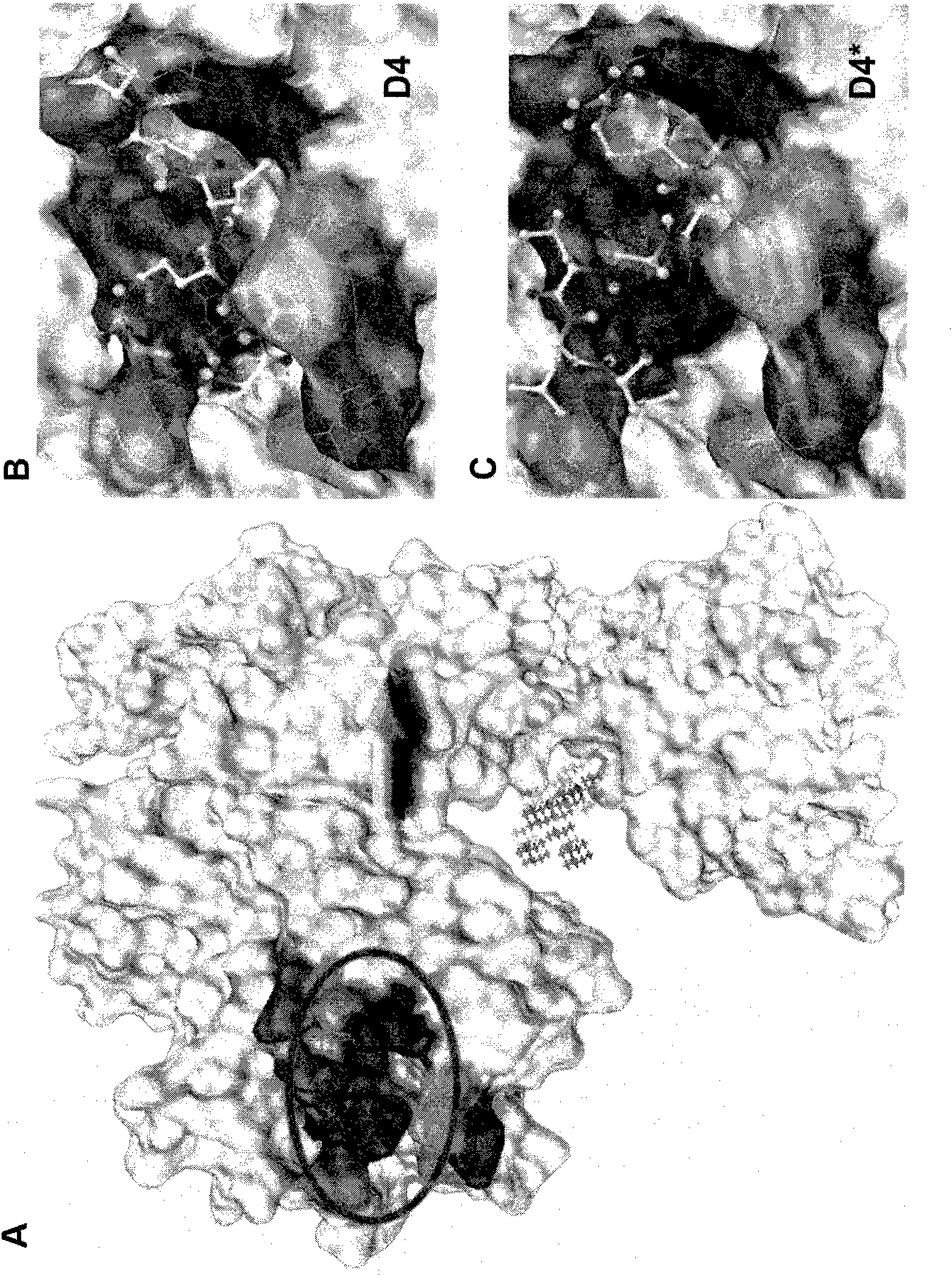
[0079] The crystal structure of EGFR in the inactive state was downloade...
Embodiment 2
[0086] Peptide LARLLT was synthesized and purified by GL Biochem Ltd. (Shanghai), and the structure and purity were confirmed by HPLC and MS. For comparison, also synthesized named D4 * A peptide with the same amino acid composition as LARLLT, but with a scrambled sequence (RTALLL, control peptide (SEQ ID NO: 2)). To fluorescently label the two peptides, Cy5.5-NHS was mixed with LARLLT or control peptide at a molar ratio of 1:2 and incubated overnight at 25°C, protected from light. The fluorescent peptide used was not further purified.
Embodiment 3
[0088] EGF or LARLLT / control peptides were dissolved in PBS-EDTA and mixed with N-succinimide-3-(2-pyridyldithio)-propionate (SPDP) dissolved in DMSO at room temperature at 1 : 1.2 molar ratio was mixed for 1 hour and then lyophilized. At the same time, the DSPE-PEG2000-Mal lipid in chloroform was evaporated to dryness to form a thin film, and hydrated in HEPES buffer (pH 7.4) at a concentration of approximately 0.4 mM. For conjugation, thioated proteins or peptides were added to tris(2-carboxyethyl)phosphine (TCEP) solution, incubated for 1 hour at room temperature under nitrogen, and rapidly mixed with MAL-PEG2000-DSPE micellar solution to A molar ratio of 5:1 was mixed and the reaction was kept stirring overnight at 10°C under nitrogen. By HPLC analysis, almost all Mal-PEG-DSPEs were modified after this reaction. Excess protein / peptide can be removed using standard techniques, eg, through gel filtration columns, dialysis, etc.
PUM
| Property | Measurement | Unit |
|---|---|---|
| phase transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com