Method for synthesizing and refining quinclorac
A technology of quinclorac and a synthesis method, applied in the field of pharmaceutical compounds, can solve the problems of complicated purification, low yield, poor product quality and the like, and achieve the effects of improving reaction yield, high yield and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
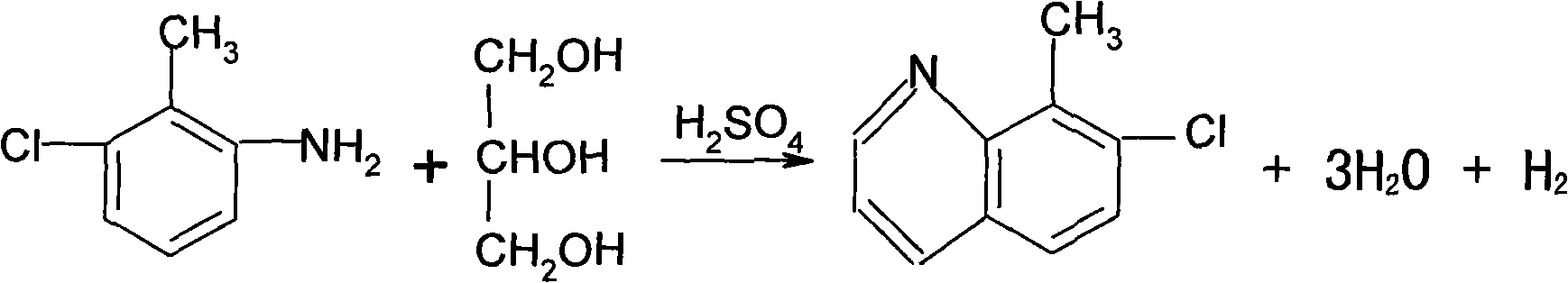
[0026] First add 125mL of water in the reaction flask A, and add 560g of concentrated sulfuric acid (98%) dropwise under stirring. After the dropwise addition is completed, start to add 250g of 3-chloro-2-methylaniline, 2g of potassium iodide, and start to add dropwise of 185g of glycerin. Generally, the dropwise addition ends in 3 hours, and the dropping temperature is kept at 148-150°C. Keep warm for 2.5 hours, add 400 mL of water after the heat preservation, cool down to below 40°C, add petroleum ether (boiling range of 60-90°C) 1000ml. Start adding dilute lye (32%) dropwise. When the pH reaches 9-10, stop the dropwise addition, and separate the layers after standing for half an hour. The upper material is filtered, the filter cake is washed with 200ml of petroleum ether, the filtrates are combined, and distilled at atmospheric pressure until no fraction comes out. After the distillation was finished, 1000 ml of o-dichlorobenzene was added for use in the next step of chlor...
Embodiment 2
[0031] First add 125mL of water in the reaction flask A, and add 560g of concentrated sulfuric acid (98%) dropwise under stirring. After the dropwise addition is completed, start to add 250g of 3-chloro-2-methylaniline, 2g of potassium iodide, and start to add dropwise of 185g of glycerol. Generally, the dropwise addition ends in 3 hours, and the dropping temperature is kept at 148-150°C. Keep warm for 2.5 hours, add 400 mL of water after the heat preservation, cool down to below 40°C, add petroleum ether (boiling range of 60-90°C) 1000ml. Start adding dilute lye (32%) dropwise. When the pH reaches 9-10, stop the dropwise addition, and separate the layers after standing for half an hour. The upper material is filtered, the filter cake is washed with 200ml of petroleum ether, the filtrates are combined, and distilled at atmospheric pressure until no fraction comes out. After the distillation was finished, 1000 ml of o-dichlorobenzene was added for use in the next step of chlor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com