Aildenafil citrate crystal form O, preparation method and application thereof
A technology of citric acid and amorphous form, which is applied in the field of edenafil citrate crystal form O and its preparation and application, and can solve problems not related to edenafil citrate crystal form and its preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
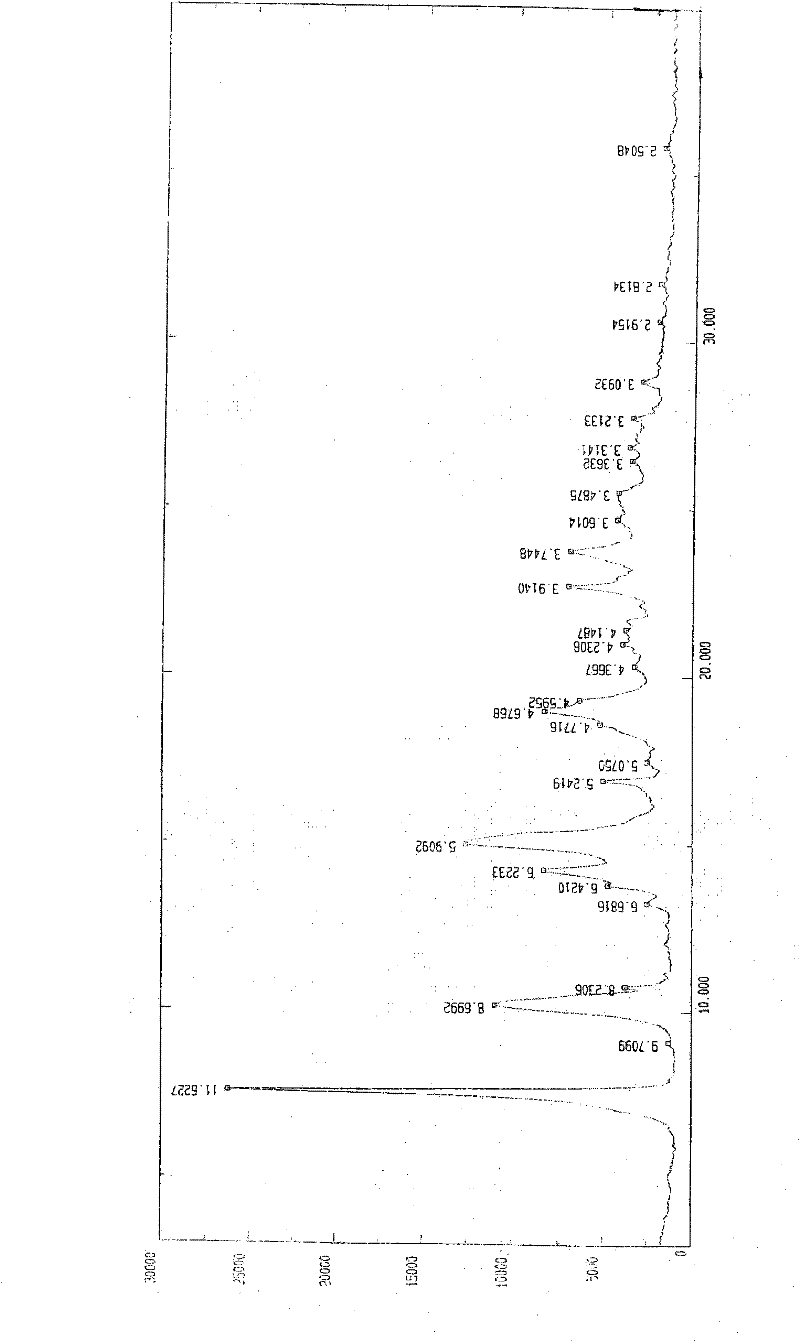
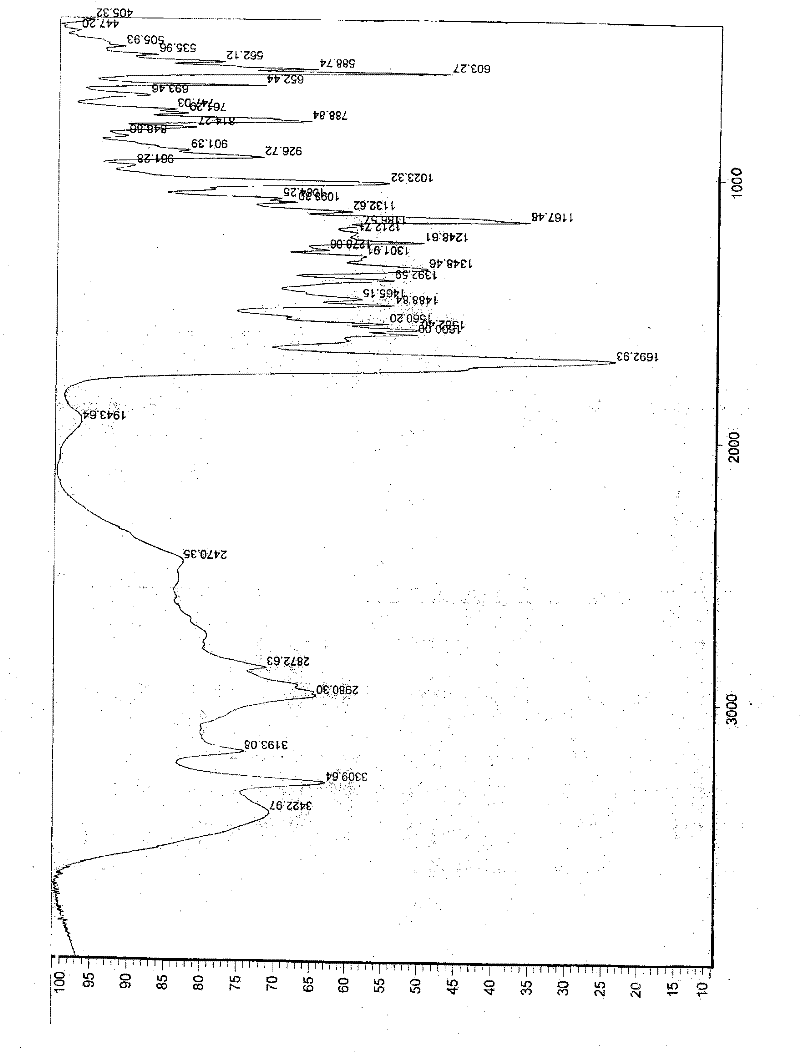
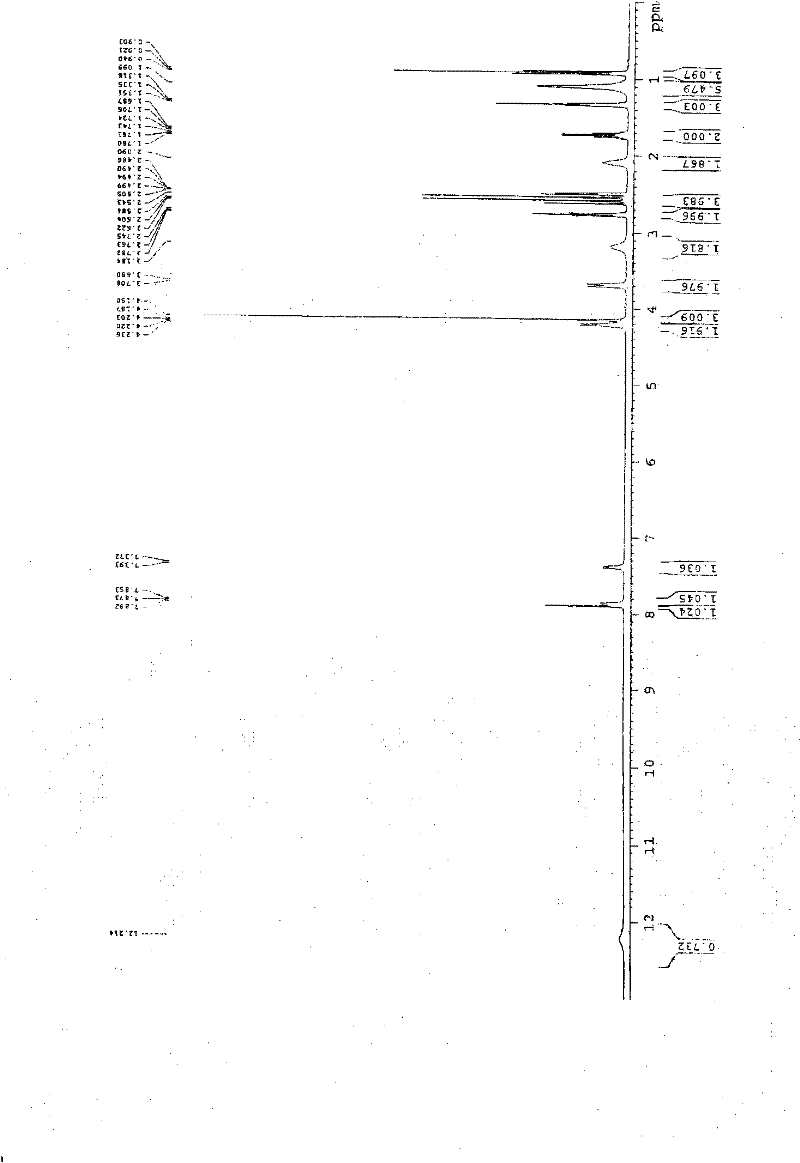
[0057] In a 1000ml reaction bottle, add 20 grams of edenafil citrate, 450ml of distilled water, and 50ml of tetrahydrofuran, start stirring, heat up to reflux, filter while it is hot after 15 minutes, stir the filtrate and cool it down to room temperature, and keep stirring for 25 hours. Precipitate crystals, filter, place indoors for 1 hour, then move to a vacuum drying oven, and vacuum dry for 3 hours to obtain 18.8 g of aldenamorph citrate crystal form O, with a purification rate of 94%, measured by HPLC area normalization method The content is 99.96%. Detected by X-ray diffractometer and infrared spectrometer (see figure 1 , figure 2 ), showing the characteristics of Aldenafil Citrate Form O.
Embodiment 2
[0059] In a 2000ml reaction bottle, add 40 grams of edenafil citrate, 900ml of distilled water, and 95ml of tetrahydrofuran, start stirring, heat up to reflux, filter while it is hot after 20 minutes, stir the filtrate and cool it down to room temperature, and keep stirring for 25 hours. Precipitate crystals, filter, place indoors for 1 hour, then move to a vacuum drying oven, and vacuum dry for 4 hours to obtain 37.6 g of aldenafil citrate crystal form O, with a purification rate of 94%, measured by HPLC area normalization method The content is 99.96%. Detected by X-ray diffractometer and infrared spectrometer (see figure 1 , figure 2 ), showing the characteristics of Aldenafil Citrate Form O.
Embodiment 3
[0061] Granules containing Aldenafil Citrate Form O
[0062] Prescription: 50 grams of Aldenafil Citrate Form O, 650 grams of lactose, 100 grams of crospovidone, 90 grams of PEG-4000, 135 grams of hydroxypropyl methylcellulose, appropriate amount of distilled water, made into 1000 bags.
[0063] Process: PEG-4000 and aldenafil citrate crystal form O are pulverized together, passed through an 80-mesh sieve, mixed with other materials, made into soft materials with distilled water, granulated, dried at low temperature, and then packed into granules.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com