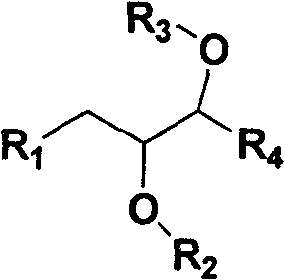
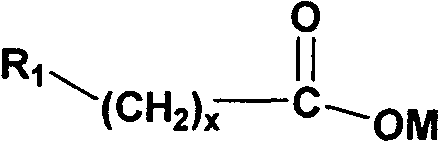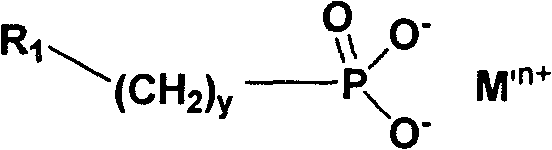Water soluble triazole compound and synthesis method thereof
A compound and triazole technology, applied in the field of water-soluble triazole compounds and the preparation of the compound, can solve problems such as safety risks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] (1) 4-[2-(2,4-difluorophenyl)-1,3-bis(1H-1,2,4-triazol-1-yl)-2-propoxy]-butanoic acid Synthesis of Ethyl Ester
[0084]
[0085] Add 30.6g (0.1mol) of 2-(2,4-difluorophenyl)-1,3-bis(1H-1,2,4-triazol-1-yl)-2-propanol into the three-necked flask (fluconazole), 27.2g (1.3mol) of ethyl 4-bromobutyrate, 2.5g of n-Bu4Br, 5mL of triethylamine, and 100ml of ethanol. Under stirring, the temperature was raised to reflux for 5 hours, the temperature was lowered, 100 mL of water was added to the reaction mixture, stirred, extracted with 150 mL of ethyl acetate x 2. Concentrate to dryness under reduced pressure, and separate by column chromatography. 17.6 g of yellow oil was obtained, yield: 42.0%.
[0086] (2) 4-[2-(2,4-difluorophenyl)-1,3-bis(1H-1,2,4-triazol-1-yl)-2-propoxy]-butanoic acid Sodium synthesis
[0087]
[0088] Add 17.6 g (0.042 mol) of the above-mentioned oily substance, 1.8 g (0.045 mol) of sodium hydroxide, 75 ml of water, and 50 ml of ethanol into the t...
Embodiment 2
[0090] (1) 2-[2-(2,4-difluorophenyl)-1,3-bis(1H-1,2,4-triazol-1-yl)-2-propoxyl]-ethyl acetate Synthesis of esters
[0091]
[0092] Add 30.6g (0.1mol) of 2-(2,4-difluorophenyl)-1,3-bis(1H-1,2,4-triazol-1-yl)-2-propanol into the three-necked flask (fluconazole), 15.9g (1.3mol) of ethyl 4-chloroacetate, 2.2g of n-Bu4Br, 5mL of triethylamine, and 100ml of ethanol. Under stirring, the temperature was raised to reflux for 5 hours, the temperature was lowered, 100 mL of water was added to the reaction mixture, stirred, extracted with 150 mL of ethyl acetate x 2. Concentrate to dryness under reduced pressure, and separate by column chromatography. 19.6 g of yellow oil was obtained, yield: 50.1%.
[0093] (2) 4-[2-(2,4-difluorophenyl)-1,3-bis(1H-1,2,4-triazol-1-yl)-2-propoxy]-sodium acetate Synthesis
[0094]
[0095] Add 19.6 g (0.05 mol) of the above-mentioned oily substance, 2.1 g (0.053 mol) of sodium hydroxide, 80 ml of water, and 55 ml of ethanol into the three-necked...
Embodiment 3
[0097] (1) 4-[(2R, 3S)-2-(2,4-difluorophenyl)-1-(1H-1,2,4-triazol-1-yl)-3-[4-( Synthesis of 4-cyanophenyl)-3-thiazolyl]-2-butoxy]-butyric acid ethyl ester
[0098]
[0099] Add 43.7g (0.1mol) of (2R,3S)-2-(2,4-difluorophenyl)-1-(1H-1,2,4-triazol-1-yl)-3 -[4-(4-cyanophenyl)-3-thiazolyl]-2-butanol, 23.3g (0.12mol) of ethyl 4-bromobutyrate, n-Bu 4 Br 2.2g, triethylamine 5mL, ethanol 120ml. Under stirring, the temperature was raised to reflux for 3 hours, the temperature was lowered, 100 mL of water was added to the reaction mixture, stirred, and extracted with 150 mL×2 ethyl acetate. Concentrate to dryness under reduced pressure, and separate by column chromatography. 23.7 g of yellow oil was obtained, yield: 43.1%.
[0100] (2) 4-[(2R, 3S)-2-(2,4-difluorophenyl)-1-(1H-1,2,4-triazol-1-yl)-3-[4-( Synthesis of 4-cyanophenyl)-3-thiazolyl]-2-butoxy]-sodium butyrate
[0101]
[0102] Take 23.7g (0.043mol) of the product from the previous step, 2.0g (0.05mol) of sodium hydr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com