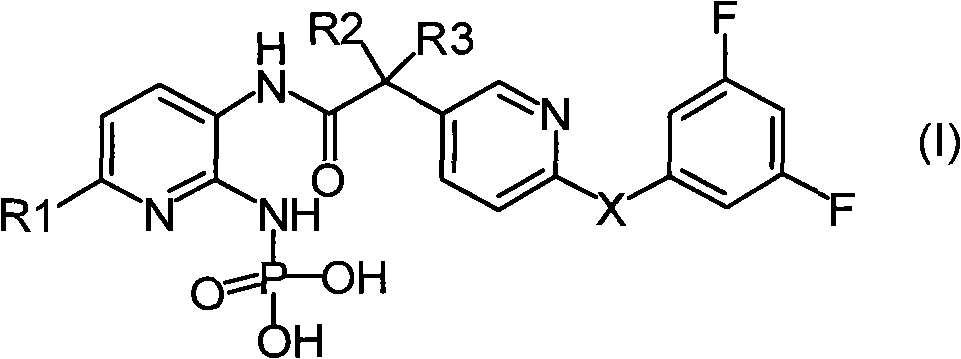
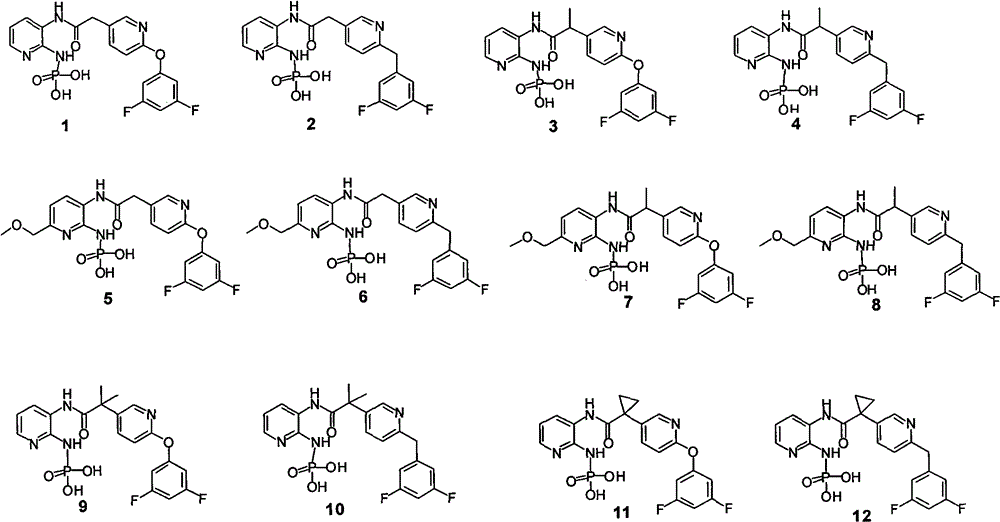
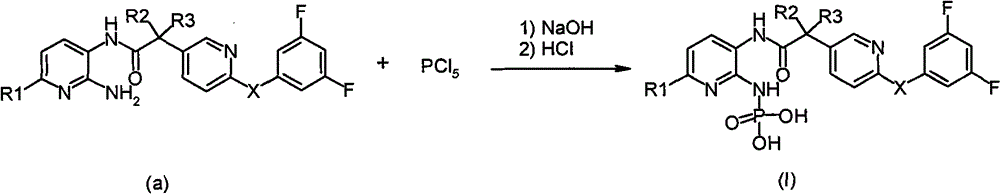
Phosphoramido pyridine derivative, and preparation method, medical composition and application thereof in antifungal agent
A technology of phosphonamidopyridine and derivatives, applied to phosphonamidopyridine derivatives, its preparation and pharmaceutical composition and application in antifungal agents, can solve the toxic and side effects of amphotericin B, limited application, resistance to Low drug rate and other problems, to achieve excellent physical and chemical properties and in vivo antifungal activity, strong inhibitory activity, and broad antifungal spectrum effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Phosphonamidopyridine Derivatives 8 and its salt Synthesis
[0059] Step 1: Compound Synthesis
[0060] Add 2-(3-(6-chloropyridine)) acetate (1.8g, 10mmol), 3,5-difluorobenzyl zinc bromide (22mL 0.5M THF solution, 11 mmol) and Pd(PPh3)4 (600 mg, 0.5 mmol) were stirred at reflux overnight. After cooling, the mixture was washed successively with water, saturated aqueous sodium bicarbonate solution and brine, concentrated to dryness, applied to ISCO combiflash and eluted with 0-20% ethyl acetate petroleum ether eluent to obtain 1.8 g of the product. MS [M+H] 278.
[0061] Step 2: Compound Synthesis
[0062] The ester (1.4g, 5mmol) obtained by the above method was dissolved in 10mL of DMF, potassium carbonate (0.7g, 5mmol) was added, methyl iodide (0.7g, 5mmol) was added dropwise, stirred at room temperature overnight, poured into 20mL of ice water, ethyl acetate Extract the ester, wash the organic phase with saturated brine, concentrate to dryness, add 10 mL of...
Embodiment 2
[0079] Phosphonamidopyridine Derivatives 11 and its magnesium salt Synthesis
[0080] Step 1: Compound Synthesis
[0081] 2-(3-(6-Chloropyridine)) acetate (18.5 g, 100 mmol) was added to 3,5-difluorophenol (14.3 g, 110 mmol) and potassium tert-butoxide (12.5 g, 110 mmol) in DMF (200mL) solution, heated and stirred at 70°C for 6 hours, cooled and poured into 500mL of ice water, filtered, the solid was washed with saturated ice-sodium carbonate aqueous solution, the solid was recrystallized with ethyl acetate, and dried to obtain 22g. MS [M+H] 280.
[0082] Step 2: Compound Synthesis
[0083] The 2-(6-(3,5-difluorophenoxy)pyridyl)methyl acetate (2.8g, 10mmol) obtained above was dissolved in 50mL of acetonitrile, 1.4g of potassium carbonate (2.8g, 20mmol) was added, and stirred for 15 Minutes later, 1,2-dibromoethane (1.9 g, 10 mmol) was added, stirred and refluxed for 3 hours, cooled and filtered, and the filtrate was concentrated to dryness. 1.5 g of the residue was ...
Embodiment 3
[0091] Preparations for Injection
[0092] components
weight
10mg
50mg
Water for Injection
q.s
[0093] Preparation method: Dissolve the active ingredient and sodium chloride in an appropriate amount of water for injection, filter the resulting solution, and fill it into containers (ampoules, infusion containers, etc.) under aseptic conditions.
[0094] The active ingredients specifically refer to phosphonamidopyridine derivatives 1-12 and salts thereof.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com