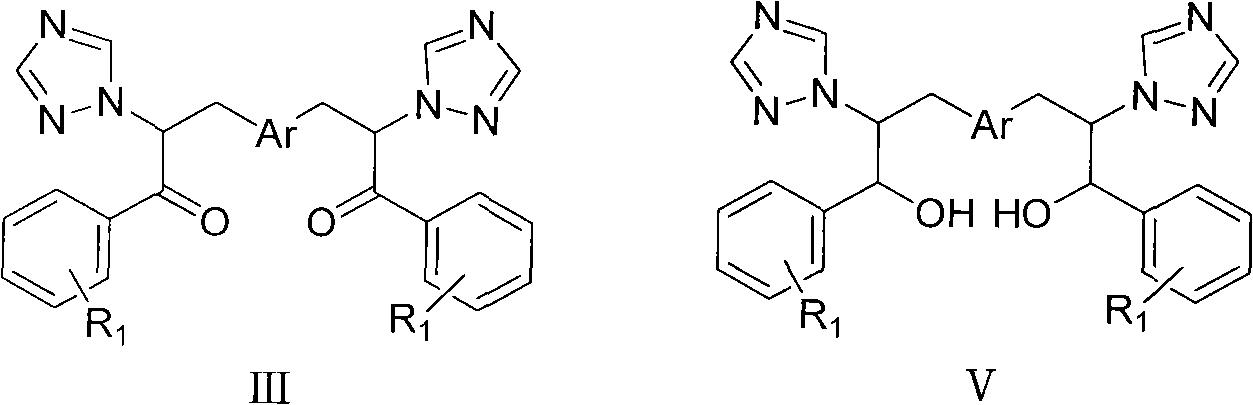
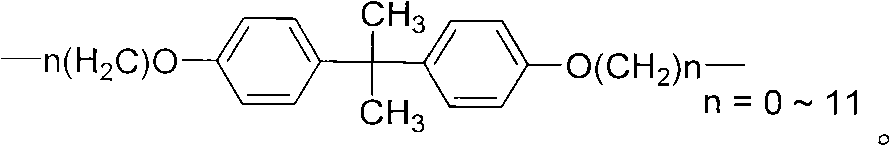
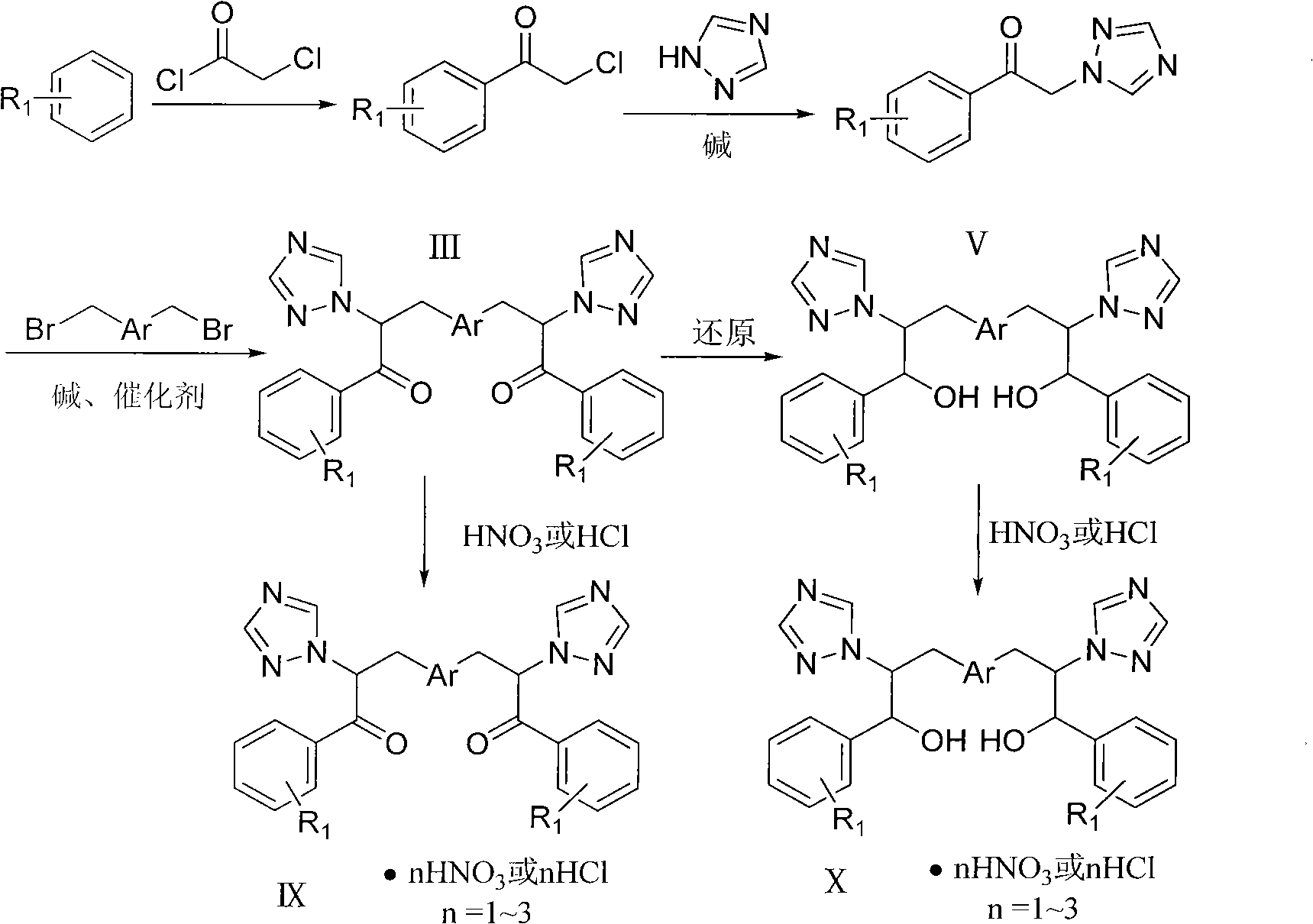
Bistriazolone, bistriadimenol compounds with antimicrobial activity, and salts, synthesis method and uses thereof
An anti-microbial, compound technology, applied in the direction of organic active ingredients, chemicals for biological control, botanical equipment and methods, etc., can solve problems such as serious drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1: Compound I-1: 3-(2,4-dichlorophenyl)-1-(2,4-difluorophenyl)-2-(1H-1,2,4-triazole)acetone
[0043] (0.88g, 0.004mol) 1-(2,4-difluorophenyl)-2-(1H-1,2,4-triazole) ethanone, potassium carbonate (0.70g, 0.005mol), tetrabutyl Ammonium bromide (25 mg), 20 mL of tetrahydrofuran were placed in a 150 mL three-necked round bottom flask, and stirred at room temperature for 1 h. (1.45 g, 0.007 mol) 2,4-dichlorobenzyl chloride was added, and the temperature of the oil bath was controlled at 45° C. and stirred for 8 h. During the reaction process, the acidity of the reaction solution was constantly checked to keep the pH value greater than 9. Use thin-layer chromatography (developer: chloroform / acetone 10 / 1, V / V) to follow the reaction. After the reaction is stopped, distill off tetrahydrofuran, extract with chloroform (20mL×3), dry over anhydrous sodium sulfate, and concentrate the mother liquor to obtain the initial product , silica gel column chromatography (developer...
Embodiment 2
[0058] Example 2: Compound II-1: 2-(2,4-dichlorophenyl)-3-(2,4-dichlorophenyl)-1-(2,4-difluorophenyl)-2- (1H-1,2,4-triazole)acetone
[0059] 1.17g, (0.005mol) 1-(2,4-difluorophenyl)-2-(1H-1,2,4-triazole) ethanone, sodium hydroxide (0.63g, 0.015mol), four Butylammonium bromide 50mg, 10mL tetrahydrofuran and 10mL H 2 O was placed in a 250mL three-neck round bottom flask and stirred at room temperature for 1h. (2.45 g, 0.013 mol) 2,4-dichlorobenzyl chloride was added, and the temperature of the oil bath was controlled at 45° C. and stirred for 6 h. During the reaction process, the acidity of the reaction solution was constantly checked to keep the pH value greater than 9. Use thin-layer chromatography (developer: chloroform / acetone 10 / 1, V / V) to track the reaction. When the reaction is complete, distill off tetrahydrofuran, extract with chloroform (20mL×3), dry over anhydrous sodium sulfate, filter, and concentrate the mother liquor to obtain 3.64 g of the initial product was...
Embodiment 3
[0072] Example 3: Compound III-1: 3,3'-(1,4-phenyl)bis(1-(2,4-difluorophenyl)-2-(1H-1,2,4-triazole )acetone
[0073] 1.17g (0.005mol) 1-(2,4-difluorophenyl)-2-(1H-1,2,4-triazole) ethanone, sodium hydroxide (0.63g, 0.015mol), tetrabutyl 50 mg of ammonium bromide, 10 mL of benzene and 10 mL of water were placed in a 250 mL three-necked round bottom flask, and stirred at room temperature for 1 h. Add (2.45 g, 0.013 mol) p-dibenzyl bromide, and stir in an oil bath at 45° C. for 6 h. During the reaction process, the acidity of the reaction solution was constantly checked to keep the pH value greater than 9. Use thin-layer chromatography (developing solvent: chloroform / acetone 10 / 1, V / V) to track the reaction. When the reaction is complete, evaporate the solvent under reduced pressure, extract with chloroform (20mL×3), dry over anhydrous sodium sulfate, filter, and the mother liquor Concentrate to obtain 3.64 g of the initial product, and perform silica gel column chromatography ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com