CuInS2 quantum dots with sphalerite structure and wurtzite structure and preparation method thereof
A technology of quantum dots and sphalerite, which is applied in the field of nanomaterials, can solve the problems of high temperature and environmentally unfriendly organic solvents, and achieve the effects of simple equipment, uniform particle size distribution, and simple preparation process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Zinc blende structure CuInS 2 Preparation method of quantum dots:
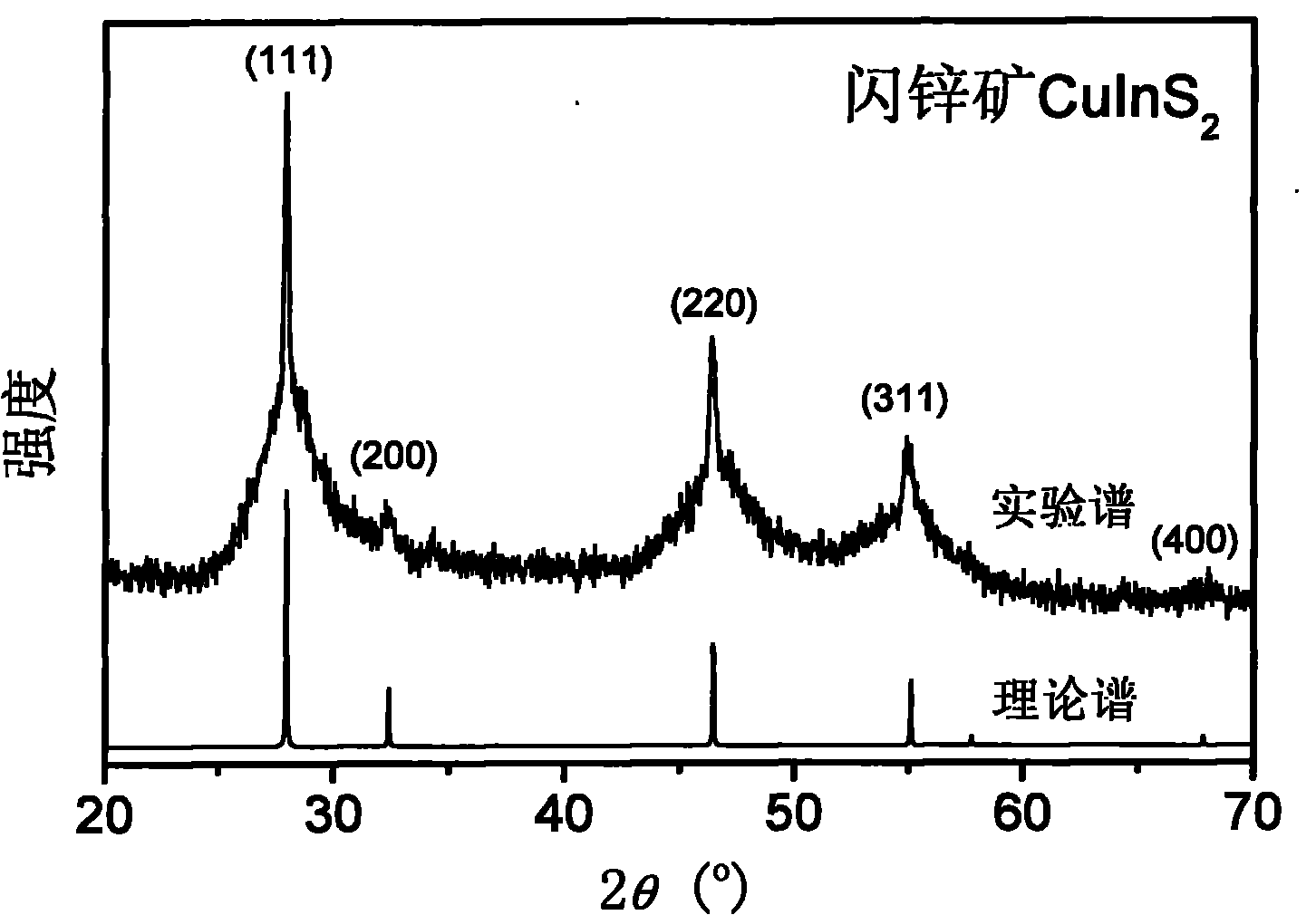
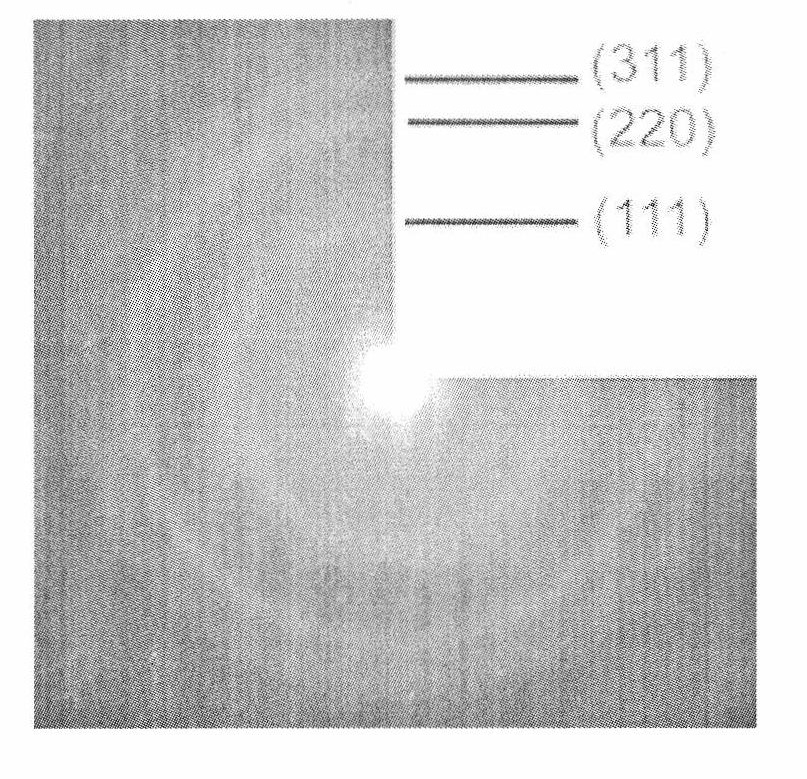
[0024] CuCl 2 (0.1mmol) and InCl 4 4H 2 O (0.1mmol) was stirred and dissolved in 35mL of absolute ethanol; 1.2mmol of p-bromothiophenol was added to the mixed solution, stirred, and a light yellow flocculent precipitate appeared in the solution; in addition, Na 2 S·9H 2 O (0.4 mmol) was sonicated in 15 mL of absolute ethanol for 10 minutes. Na 2 S solution was added to 35 mL of CuCl 2 and InCl 4 In the mixture, the solution turned reddish brown. Finally, the mixture was transferred into a 60 mL autoclave lined with polytetrafluoroethylene, and kept at 200° C. for 16 hours. After the autoclave was naturally cooled to room temperature, the upper liquid was removed to obtain a brown precipitate; the precipitate was washed with absolute ethanol and centrifuged (16000rpm, 10min), and the product was dried in a vacuum oven at 60°C for 6 hours to obtain CuInS 2 Powder composed of quantum dots.
[00...
Embodiment 2
[0027] Wurtzite CuInS 2 Preparation method of quantum dots:
[0028] CuCl 2 (0.1mmol) and InCl 4 4H 2 O (0.1mmol) was stirred and dissolved in 35mL absolute ethanol; 35mmol hexanethiol was added to the mixed solution, stirred, and a white flocculent precipitate appeared in the solution; in addition, Na 2 S·9H 2 O (0.4 mmol) was sonicated in 15 mL of absolute ethanol for 10 minutes. Na 2 S solution was added to 35 mL of CuCl 2 and InCl 4 In the mixture, the solution turned reddish brown. Finally, the mixture was transferred into a 60 mL autoclave lined with polytetrafluoroethylene, and kept at 200° C. for 16 hours. After the autoclave was naturally cooled to room temperature, the upper liquid was removed to obtain a brown precipitate; the precipitate was washed with absolute ethanol and centrifuged (16000rpm, 10min), and the product was dried in a vacuum oven at 60°C for 6 hours to obtain CuInS 2 Powder composed of quantum dots.
[0029] Powder XRD test shows that th...
Embodiment 3
[0031] Wurtzite CuInS 2 Preparation method of quantum dots:
[0032] CuCl 2 (0.1mmol) and InCl 4 4H 2 O (0.1mmol) was stirred and dissolved in 35mL absolute ethanol; 35mmol ethanethiol was added to the mixed solution, stirred, and white flocculent precipitation appeared in the solution; in addition, Na 2 S·9H 2 O (0.4 mmol) was sonicated in 15 mL of absolute ethanol for 10 minutes. Na 2 S solution was added to 35 mL of CuCl 2 and InCl 4 In the mixture, the solution turned reddish brown. Finally, the mixture was transferred into a 60 mL autoclave lined with polytetrafluoroethylene, and kept at 200° C. for 16 hours. After the autoclave was naturally cooled to room temperature, the upper liquid was removed to obtain a brown precipitate; the precipitate was washed with absolute ethanol and centrifuged (16000rpm, 10min), and the product was dried in a vacuum oven at 60°C for 6 hours to obtain CuInS 2 Powder composed of quantum dots.
[0033] Powder XRD test shows that th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com