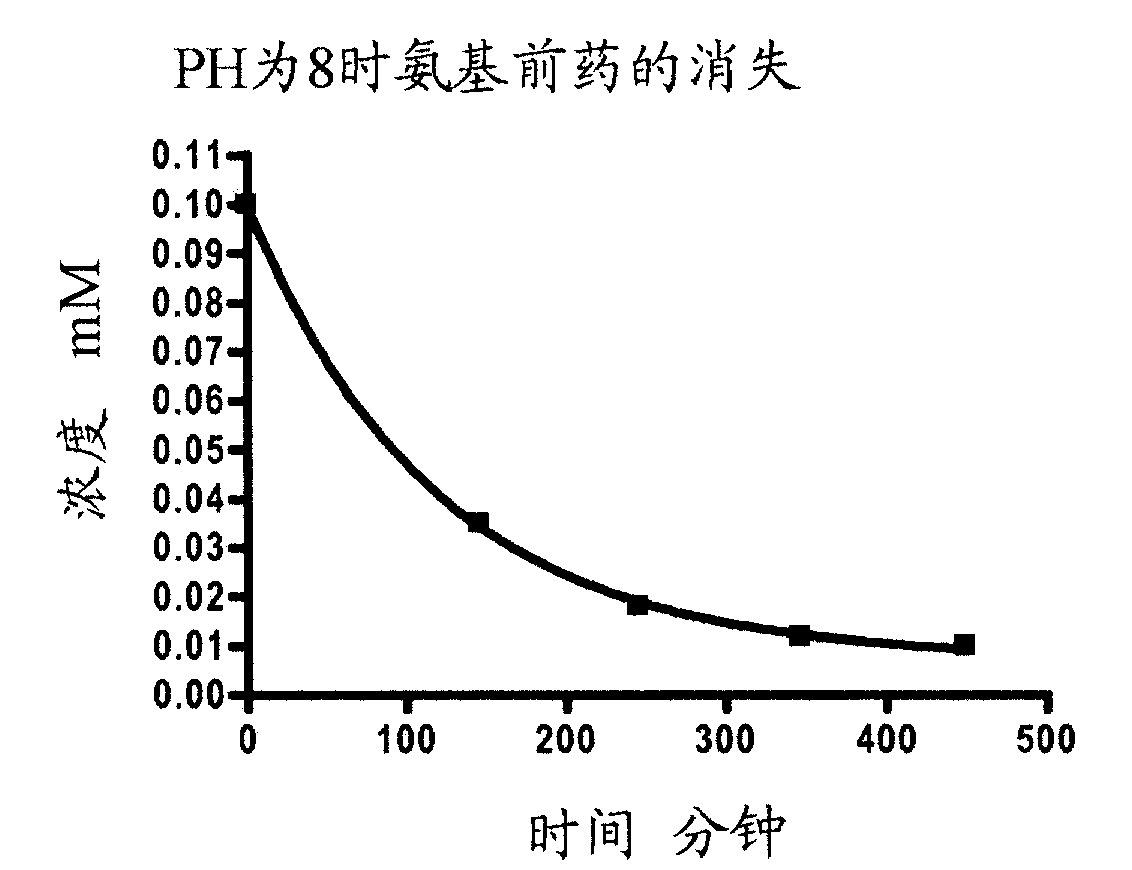
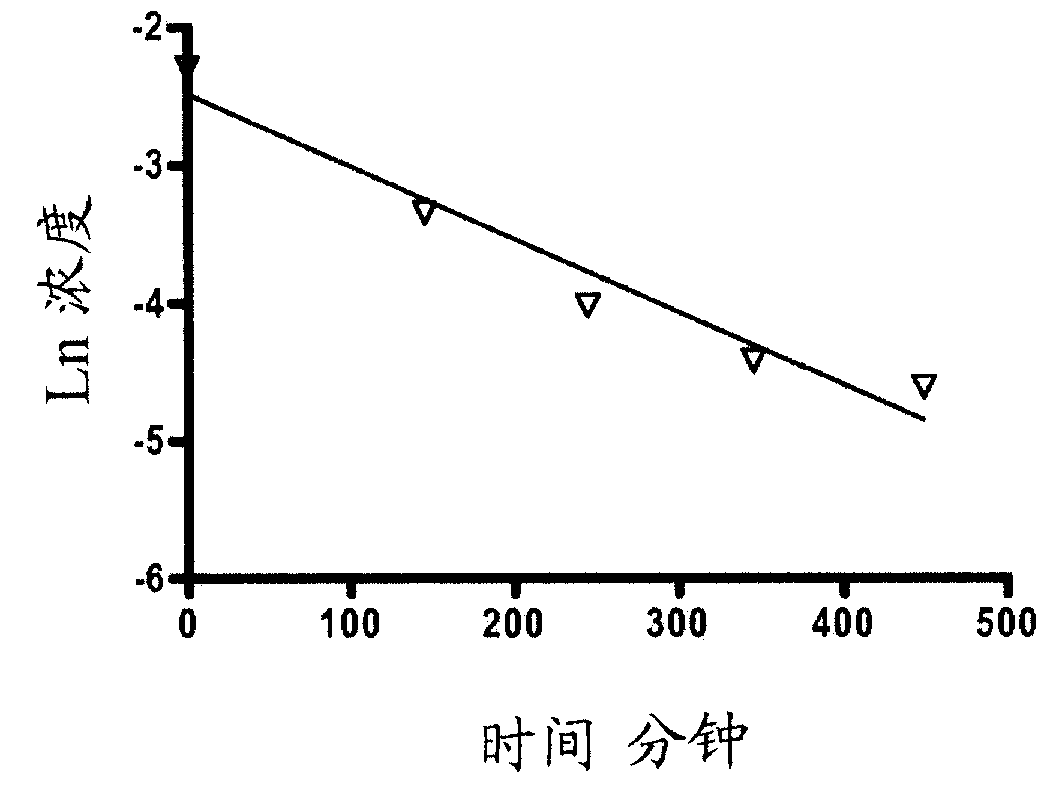
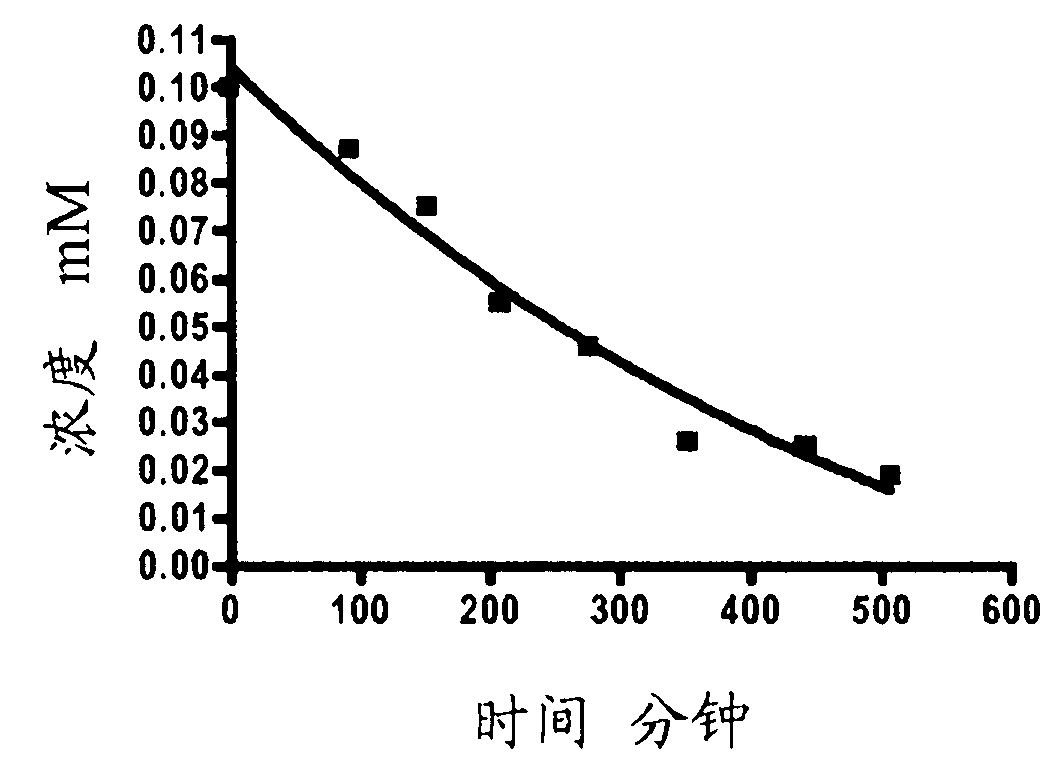
Targeting diazo prodrugs for the treatment of gastrointestinal diseases
A pharmacy and drug technology, applied in bone diseases, antipyretics, anti-infectives, etc., can solve problems such as limited success and slow release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0051] method
[0052] The following procedure describes the preparation of a series of esters of the anti-inflammatory steroids hydrocortisone, dexamethasone or budesonide from azo derivatives containing amino esters at the 21-, 11- and 17-positions.
[0053] Ring Closure Kinetic Compounds
[0054]In a specific embodiment, the synthesis method of dexamethasone is summarized as follows (Scheme 4). 2-Nitrocinnamic acid was reduced to phenylalanine by catalytic reduction and immediately treated with BOC anhydride. The most exposed steroid hydroxyl at position 21 was selectively acylated with N,N'-dicyclohexylcarbodiimide / dimethylaminopyridine (Johnson et al, 1985). The BOC protection can be removed by bubbling anhydrous HCl through a dichloromethane (DCM) solution and aniline 6 is recovered as the hydrochloride salt. The salt was stored at -80°C until use. Spontaneous ring closure of methyl 3-(2-aminophenyl)propionate occurs even when stored at -20°C (Kirby et al., 1979).
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com