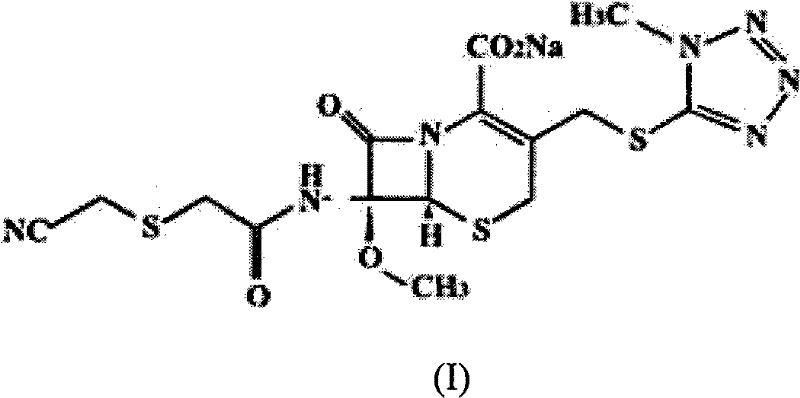
Method for preparing cefmetazole sodium
A technology of cefmetazole sodium and cefmetazole acid, which is applied in the field of preparation of cephalosporins, can solve the problems of low yield and product purity, many impurities, deep color, etc., and achieve low cost and environment-friendly effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
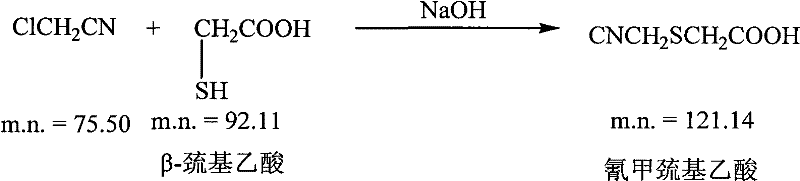
[0043] (1) Preparation of side chain (cyanomethylthioglycolic acid)
[0044] 100ml of water, cooled to 10±2°C, added 20g of sodium hydroxide until completely dissolved, then added 20g of thioglycolic acid, stirred for 10 minutes, added 15g of chloroacetonitrile, reacted for 1 hour; cooled to 10±2°C, added 16.2 g of sodium chloride kg, stir to dissolve, add 90ml of ethyl acetate, dropwise add 36% hydrochloric acid to adjust the pH to 3.5, stir for 10 minutes, stand still and separate the phases, discard the water phase, concentrate the solvent phase to 25-30ml, cool to 0°C-5°C for later use;
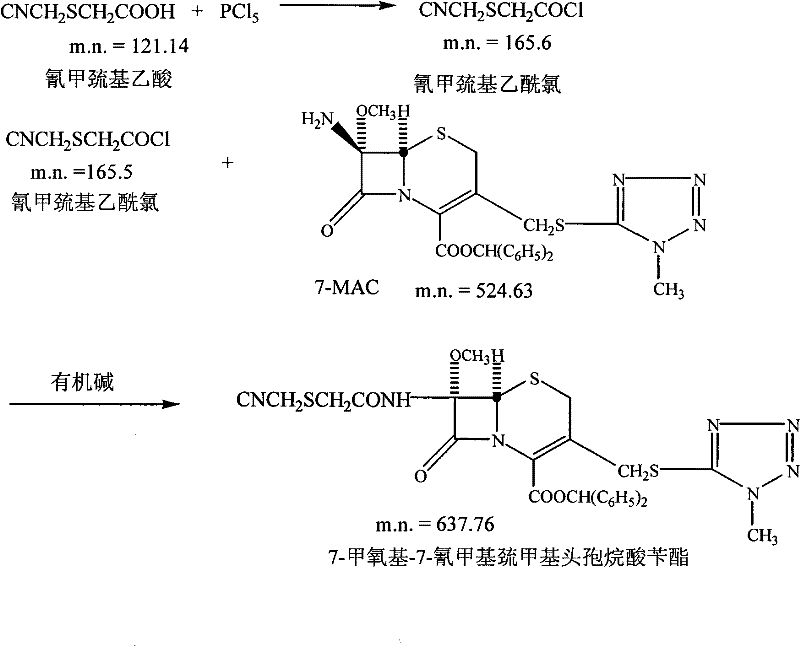
[0045] (2) preparation of cefmetazole benzyl axetil
[0046] a: 80ml of dichloromethane, add 50g of phosphorus pentachloride, keep stirring at 10±2°C for 15 minutes, add 12.5ml of side chain (cyanomethylthioglycolic acid) into the solution, timed stirring for 1 hour, set aside;
[0047] b: Add 1000ml of dichloromethane, add 15g of 7-MAC, cool down to -100°C~-10°C, add 5g of organic base,...
Embodiment 2
[0055] (1) Preparation of side chain (cyanomethylthioglycolic acid)
[0056] 100ml of water, cooled to 10±2°C, added 25g of sodium hydroxide until completely dissolved, added 35g of mercaptoacetic acid, stirred for 10 minutes, added 25g of chloroacetonitrile, reacted for 1 hour; cooled to 10±2°C, added 16.2 g of sodium chloride kg, stir to dissolve, add 100ml of ethyl acetate, add dropwise 36% hydrochloric acid to adjust the pH to 3.5, stir for 10 minutes, stand still and separate the phases, discard the water phase, concentrate the solvent phase to 25-30ml, cool to 0°C-5°C for later use;
[0057] (2) preparation of cefmetazole benzyl axetil
[0058] Add 100ml of dichloromethane, add 65g of phosphorus pentachloride, keep stirring at 10±2°C for 15 minutes, add 13ml of side chain (cyanomethylthioglycolic acid) into the solution, and stir for 1 hour to obtain a side chain solution, which is set aside;
[0059] Add 1000ml of dichloromethane, add 18g of 7-MAC, cool down to -100°C~...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com