Propofol, phosphate and amino acid double salt and preparation method and application thereof
A technology of propofol phosphate and propofol dihydrogen phosphate, applied in its preparation, water-soluble prodrug-propofol phosphate amino acid double salt, application field in the preparation of sedative hypnotic and anesthetic drugs , can solve the problems of unfavorable drug safety and achieve the effects of improving water solubility and bioavailability, less environmental pollution, and reducing irritation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
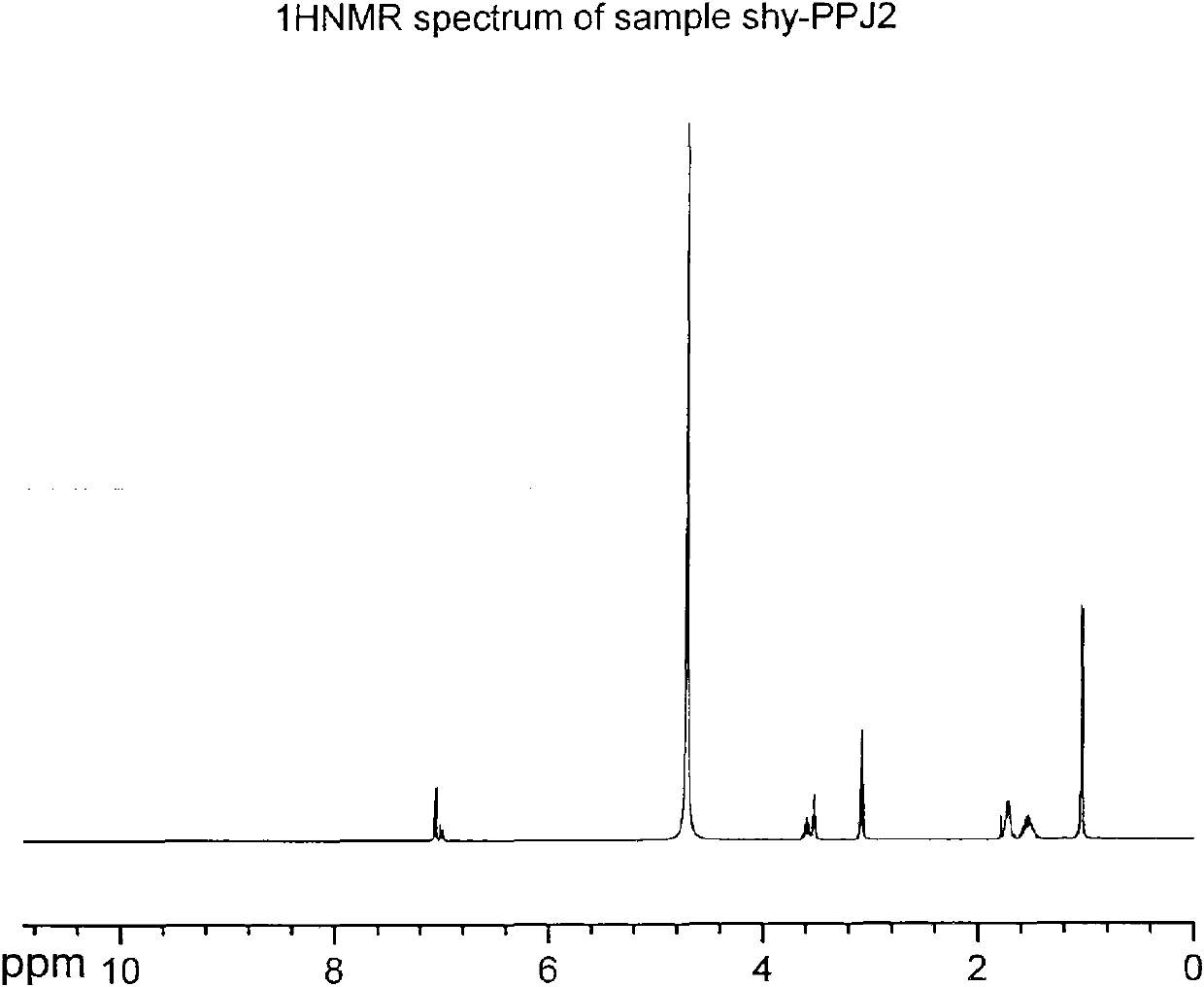
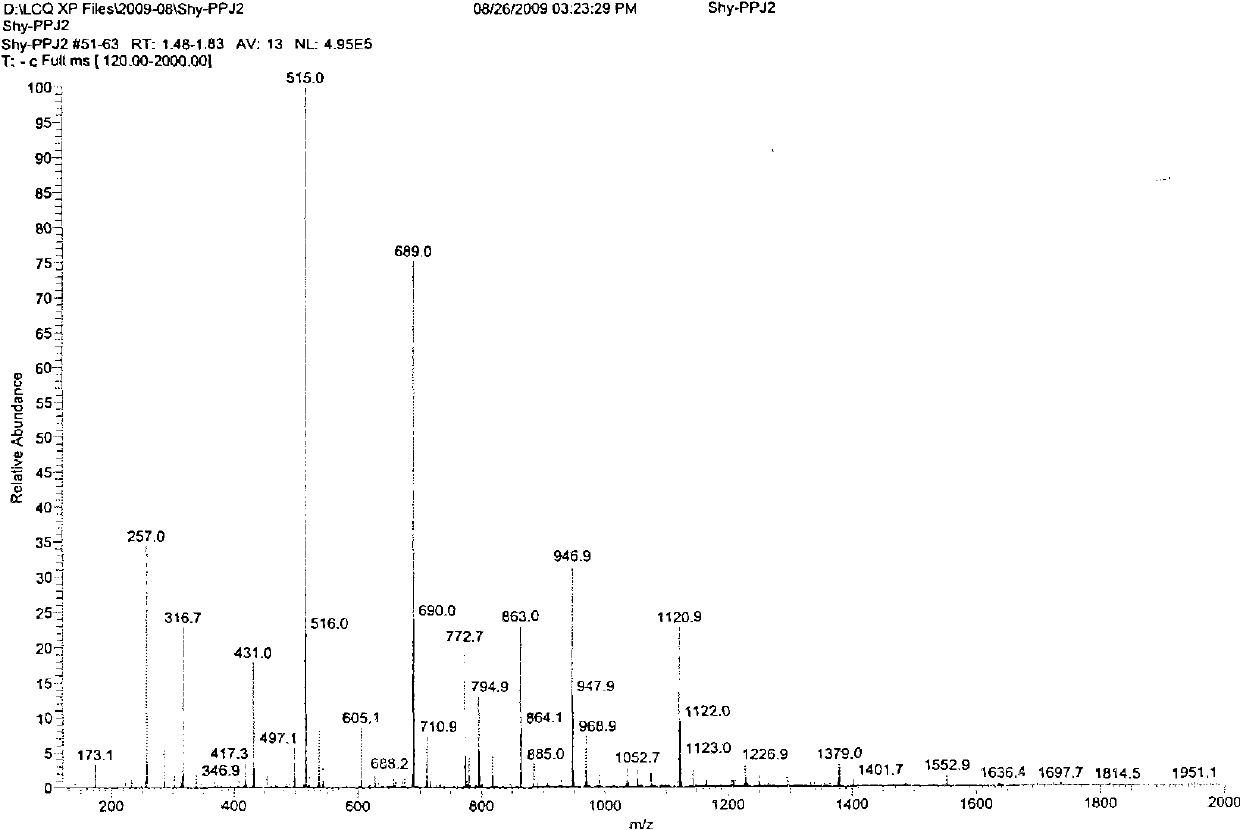
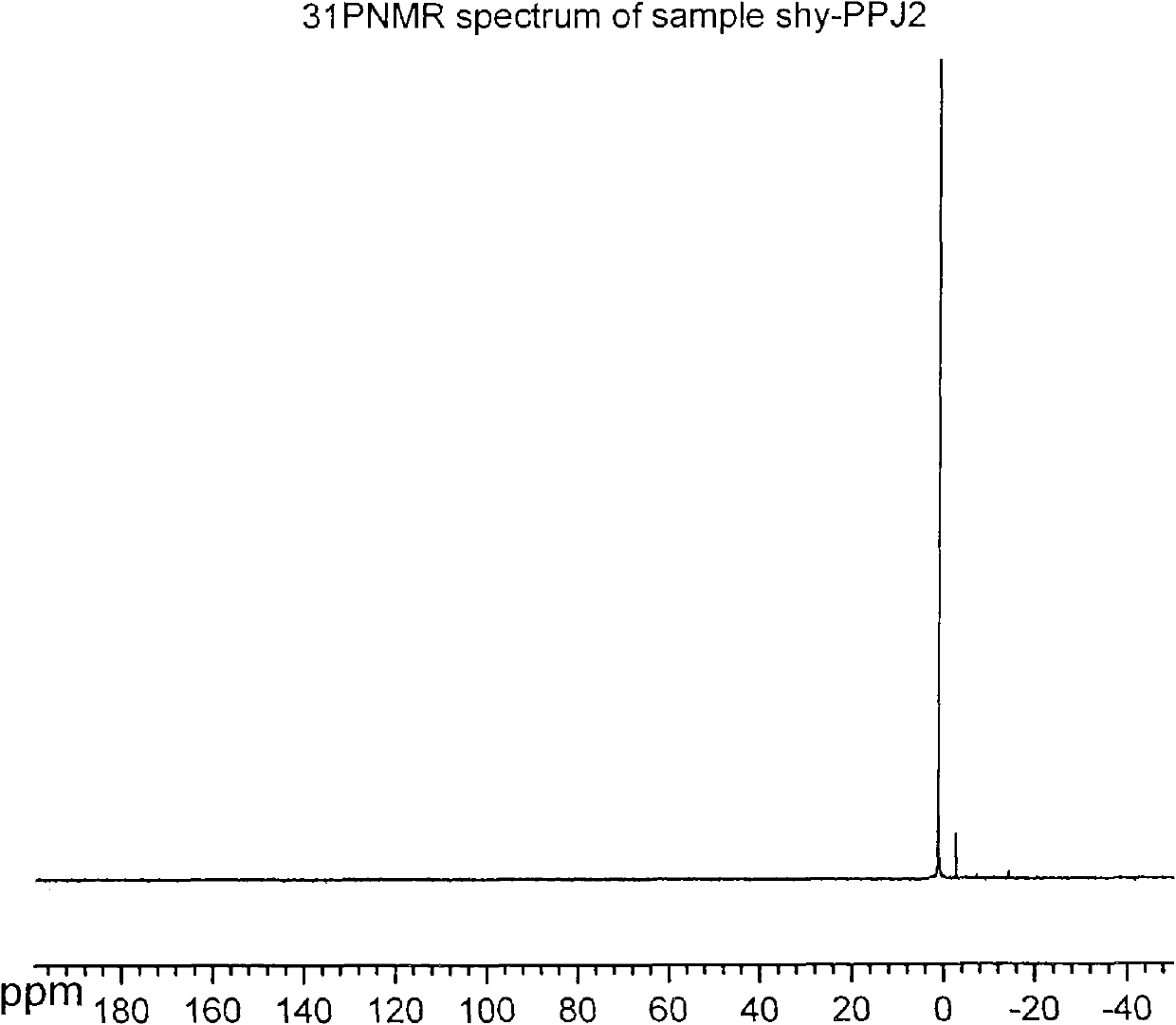
[0040] Add 1.78 g (0.01 mol) of propofol, 1.52 g (0.015 mol) of triethylamine, and 10 ml of dichloromethane into a 50 ml three-necked flask equipped with a thermometer, a constant pressure dropping funnel, and a reflux condenser. Stir the reaction at 25° C. After 0.5 hours, slowly add 2.30 g (0.015 mol) of phosphorus oxychloride dropwise, and the dropwise addition is completed in about 1 hour. Complete, add water at 25°C for hydrolysis, control the reaction solution to be always acidic, stir for 2 hours, the reaction is complete, wash with water until the water layer is basically colorless, back-extract the water layer with dichloromethane, combine the organic layers, and spin dry to obtain red Brown viscous liquid, and then purified to obtain 2.01 g of white crystals of propofol dihydrogen phosphate, yield: 77.9%.
Embodiment 2
[0042] Add 1.78g (0.01mol) of propofol, 1.52g (0.015mol) of triethylamine, and 10ml of dichloromethane into a 50ml three-necked flask equipped with a thermometer, a constant pressure dropping funnel and a reflux condenser, and stir the reaction at 0°C After 0.5 hours, slowly add 2.30 g (0.015 mol) of phosphorus oxychloride dropwise, and the dropwise addition is completed in about 1 hour. Complete, add water at 0°C for hydrolysis, control the reaction solution to always be acidic, stir and react for 4 hours, the reaction is complete, wash with water until the water layer is basically colorless, back-extract the water layer with dichloromethane, combine the organic layers, and spin dry to obtain red Brown viscous liquid, and then purified to obtain 1.53 g of white crystals of propofol dihydrogen phosphate, yield: 59.3%.
Embodiment 3
[0044]Add 1.78 g (0.01 mol) of propofol, 1.52 g (0.015 mol) of triethylamine, and 10 ml of dichloromethane into a 50 ml three-necked flask equipped with a thermometer, a constant pressure dropping funnel, and a reflux condenser. Stir the reaction at 25° C. After 0.5 hours, slowly add 2.30 g (0.015 mol) of phosphorus oxychloride dropwise, and the dropwise addition is completed in about 1 hour. Complete, add water at 50°C for hydrolysis, control the reaction solution to be always acidic, stir for 1 hour, the reaction is complete, wash with water until the water layer is basically colorless, back-extract the water layer with dichloromethane, combine the organic layers, and spin dry to obtain red Brown viscous liquid, and then purified to obtain 1.89 g of white crystals of propofol dihydrogen phosphate, yield: 73.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com