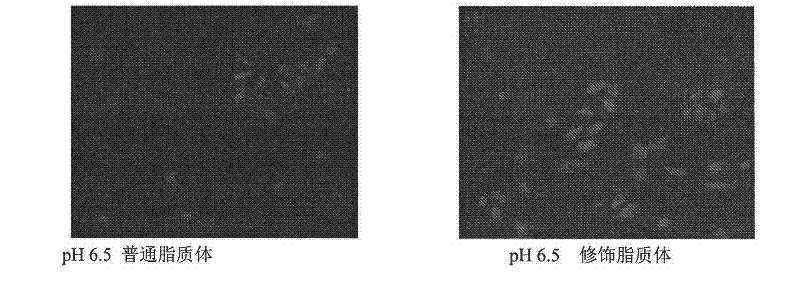
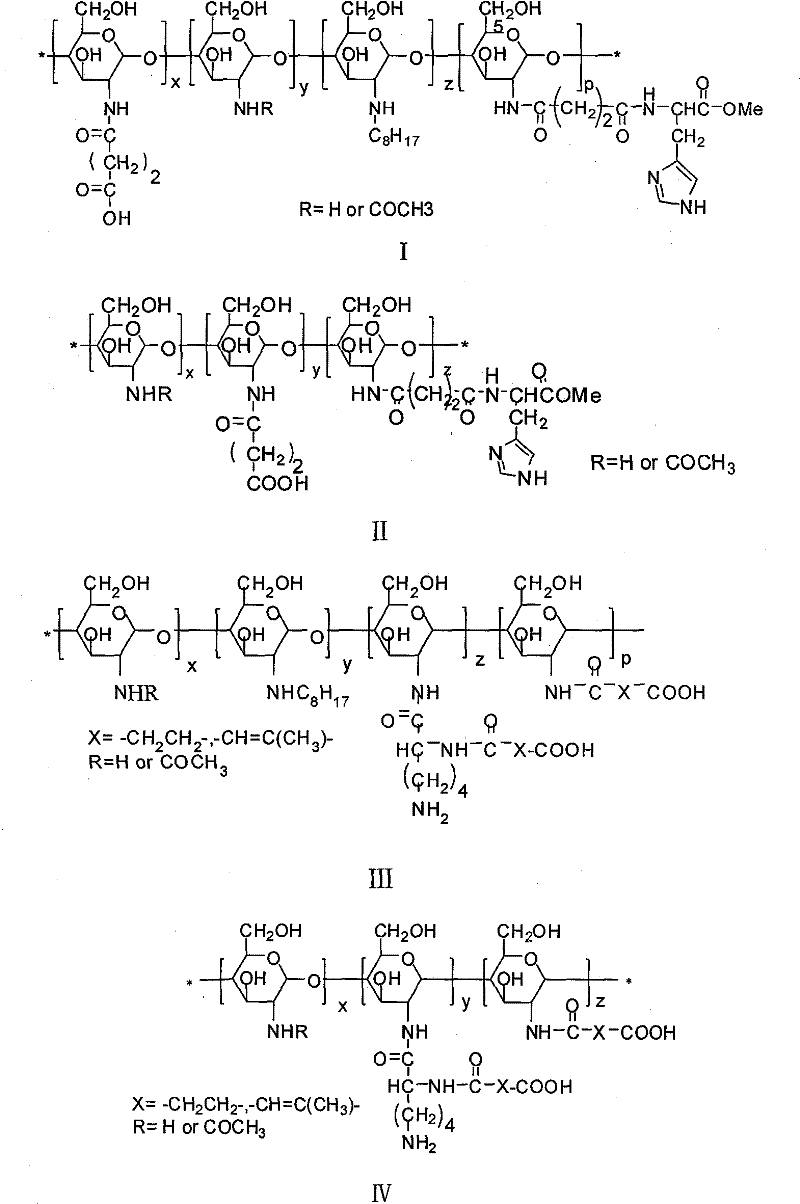
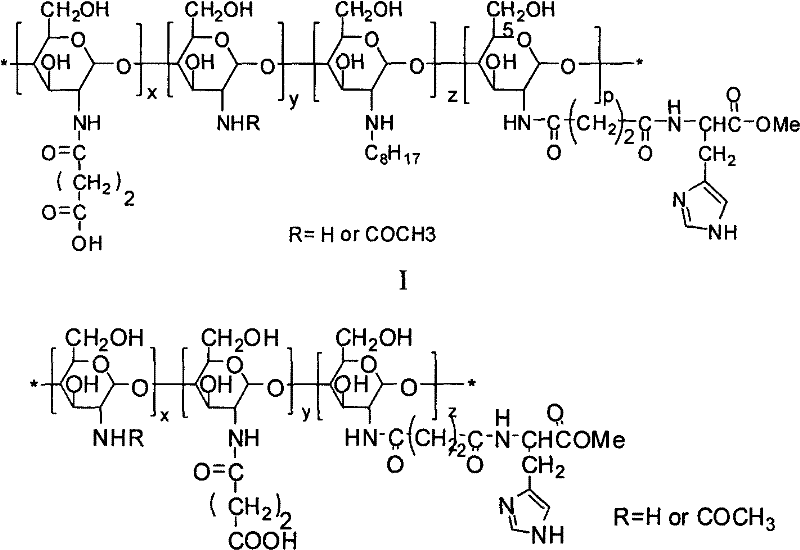
Amphoteric ion-based charge reversal chitosan derivative and application thereof in medicament
A technology of chitosan derivatives and polymer glue, applied in the field of polymer chemistry, can solve the problems of large physiological toxicity, fast plasma clearance rate, difficult application, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] 1. Preparation of N-octyl chitosan (NOC)
[0048] Add 4g of chitosan to a 500mL three-necked bottle, add 105mL of anhydrous methanol to the three-necked bottle, heat up to 30°C, and keep stirring for 2h. Add 10.6mL octanal, react at room temperature for 12h, slowly add 5g KBH 4 , reduced reaction at room temperature for 24 hours, adjusted the pH of the reaction solution to 7, filtered, washed twice with water, four times with methanol, twice with ether, and dried to obtain 4.6 g of yellow powder (octyl substitution degree 25%).
[0049] 2. Preparation of N-succinyl histidine methyl ester
[0050] Suspend 15.5g (0.1mol) of L-histidine in 320ml of anhydrous methanol, cool in an ice bath to 0°C-5°C, and slowly add 10.9ml (0.15mol) of thionyl chloride dropwise. After the dropwise addition was completed, the temperature was raised to 68° C., and the reaction was refluxed for 16 hours. After the reaction was completed, the reaction solution was concentrated to obtain a whi...
Embodiment 2
[0060] 1. N-octyl-N'-tert-butoxycarbonyl lysyl chitosan (NONLB)
[0061] Chitosan reacted with octanal, using KBH 4 Reduction, NOC was prepared according to the preparation method of NOC in Example 1. Take 1g of N-octyl chitosan (NOC) and swell in 50ml of dimethyl sulfoxide at room temperature for 12h. Add 3g (0.009mol) N, N ε - Boc-L-lysine and 1.92g (0.017mol) NHS, cooled to 0°C in an ice bath. Dissolve 3.3g (0.017mol) of EDC·HCl in 10ml of methanol and slowly drop into the reaction system. The reaction was continued for 24 h at room temperature. Reaction solution is poured in 250ml acetone, filters, filter cake water successively, water-acetone mixed liquor, acetone washing, dry to obtain 1.8g yellow powder N-octyl-N'-tert-butoxycarbonyl lysyl chitosan.
[0062]Suspend 1 g of NONLB in 20 ml of methanol and stir at room temperature for 1 h, add 20 ml of formic acid under ice-cooling, react at 0-5°C for 8 h, adjust the pH to neutral with 1 mol / L NaOH solution, dialyze fo...
Embodiment 3
[0070] 1. N-octyl-N'-citraroyl-N"-lysyl chitosan (NONLC)
[0071] Use citric anhydride to react with NONL, and the preparation method follows the preparation of NONLS in Example 2.
[0072] FT-IR: 3423, 2956, 2863, 1729, 1668, 1549, 1420, 1381, 1236, 1158, 1112, 1066, 1033cm -1 .
[0073] 1 H NMR (500MHz, D 2 O): 6.4-5.9 (-C H =C(CH 3 )-), 4.6-4.5 (HαLys, H 1 ), 4.0-3.3 (H 3 , H 4 , H 5 , H 6 ), 3.2 (-NH-C H 2 -(CH 2 ) 10 -CH 3 ), 3.0 (H 2 )2.5-2.2 (HεLys), 2.0 (NH-CO-C H 3 ), 1.7 (HβLys), 1.6-1.0 (-NH-CH 2 -(C H 2 ) 10 -CH 3 , HγLys, HδLys), 0.8(-NH-CH 2 -(CH 2 ) 10 -C H 3 ).
[0074] According to the elemental analysis data, according to the elemental analysis data, it can be calculated that the N-octyl-N'-succinyl-N"-lysyl chitosan octyl substitution degree is 27%; according to 1 The integrated area of H in H NMR can be calculated to be 49% and 41% for citroyl and lysyl, respectively.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com