Electrolytic method for controlling the precipitation of alumina
An alumina and precipitation technology, applied in chemical instruments and methods, inorganic chemistry, aluminum compounds, etc., can solve the problems of poor quality, excessive purchase cost of lime, etc., and achieve the effect of improving precipitation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
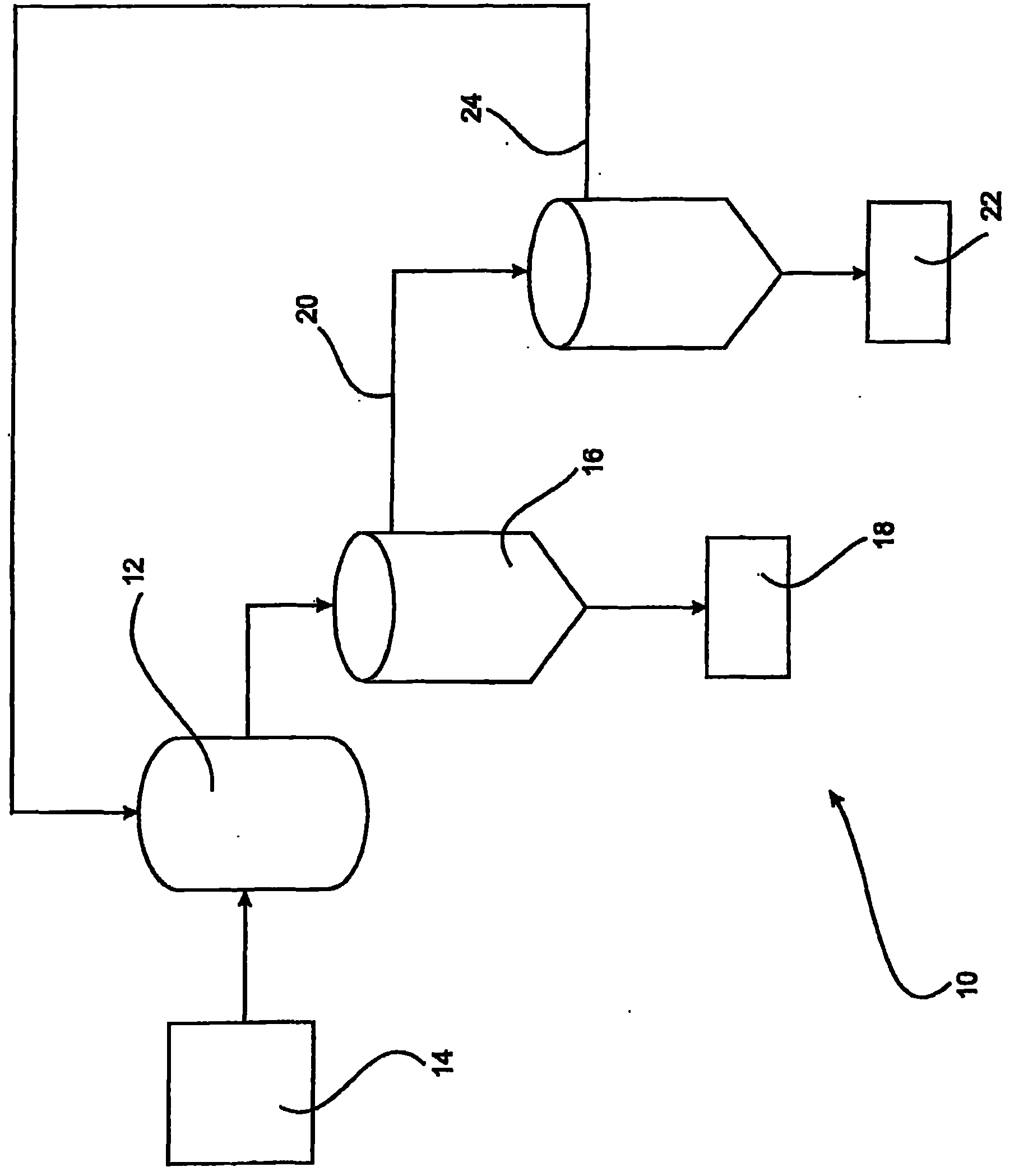
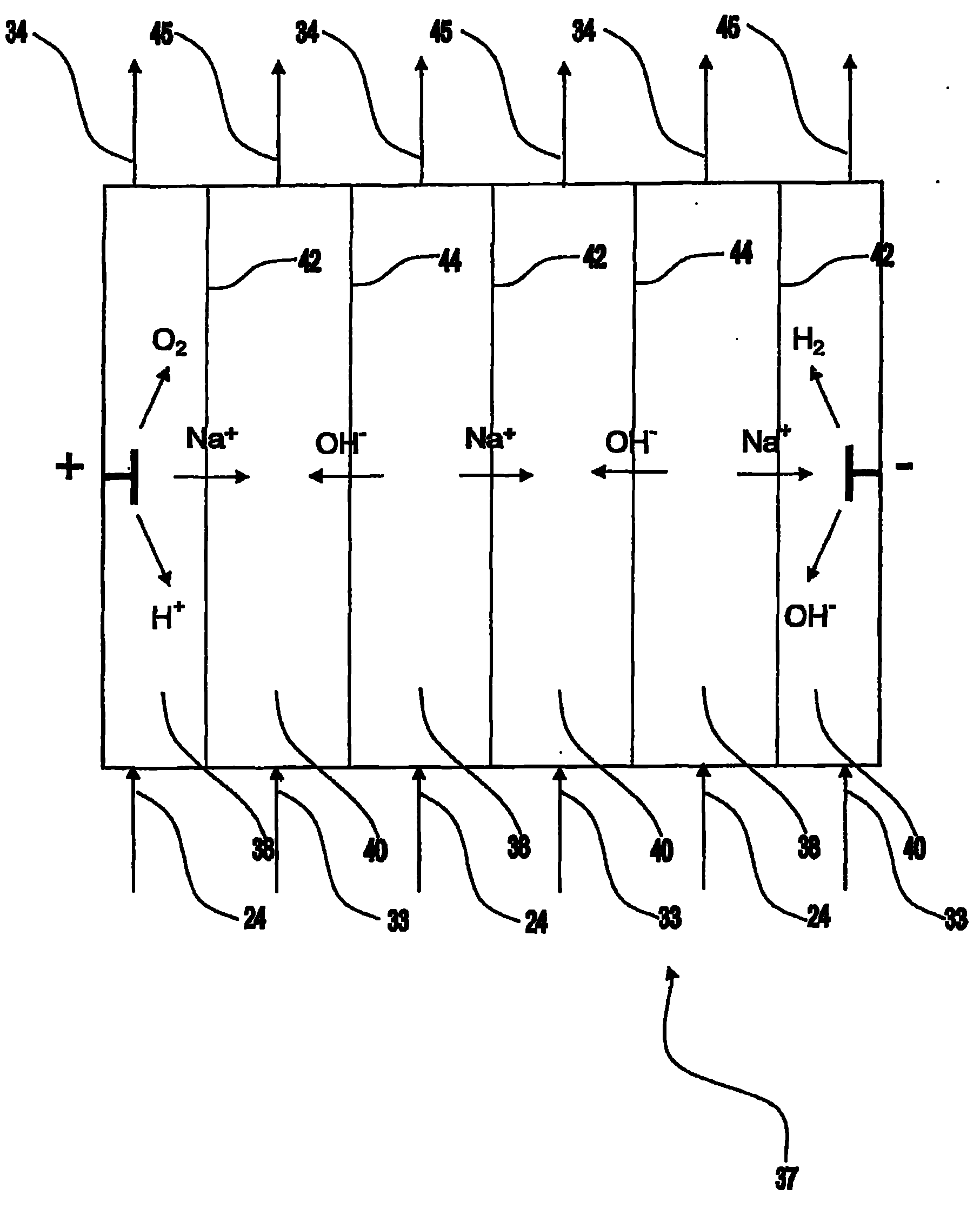
[0227] The following examples demonstrate that effluents can be supersaturated by removal of soda ash using membrane electrolysis and seeded to produce significant amounts of alumina compared to a control sample of effluent that was not subjected to prior electrolysis.
[0228] A sample of ex-precipitation liquid (LXP) with a TC of 241 (expressed as g / L of sodium carbonate) and an A / TC of 0.48 from the applicant's Kwinana refinery was divided into two parts. Some of the fractions were electrolyzed at 90°C using a Nafion 324 membrane, reducing the TC to 167 g / L and increasing the A / TC to 0.68. Both LXP and electrolyte (100 mL each) were transferred to separate polypropylene bottles, and gibbsite seeds (10 g) were added to each solution. The vial was sealed and placed in a rotating water bath at 70°C for 24 hours, after which time the solid was collected by filtration, dried at 105°C, and weighed. Both filtrates were analyzed for alumina and TC content. The results of two expe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com