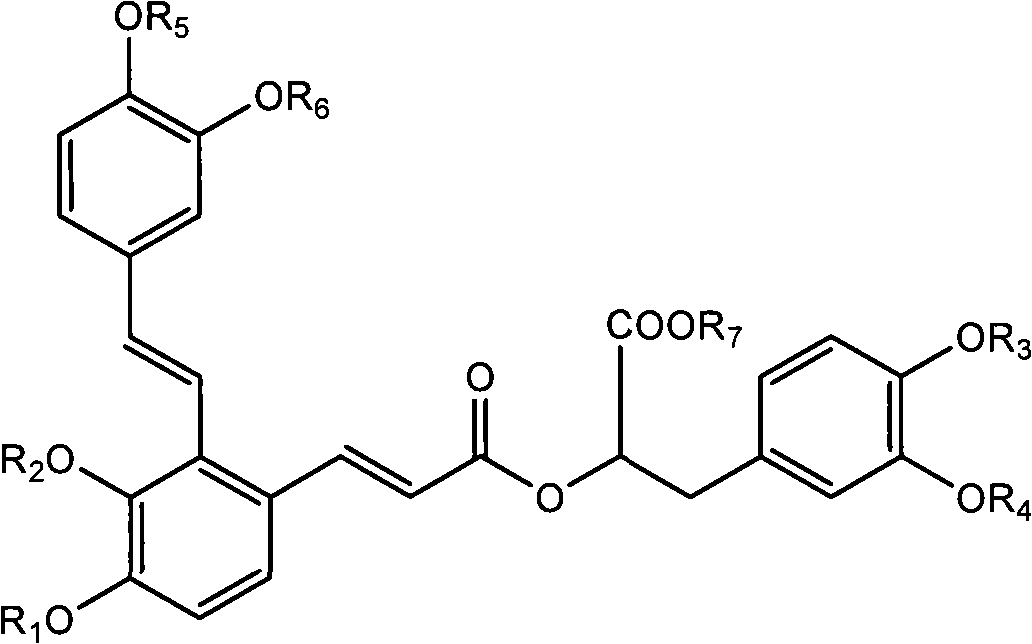
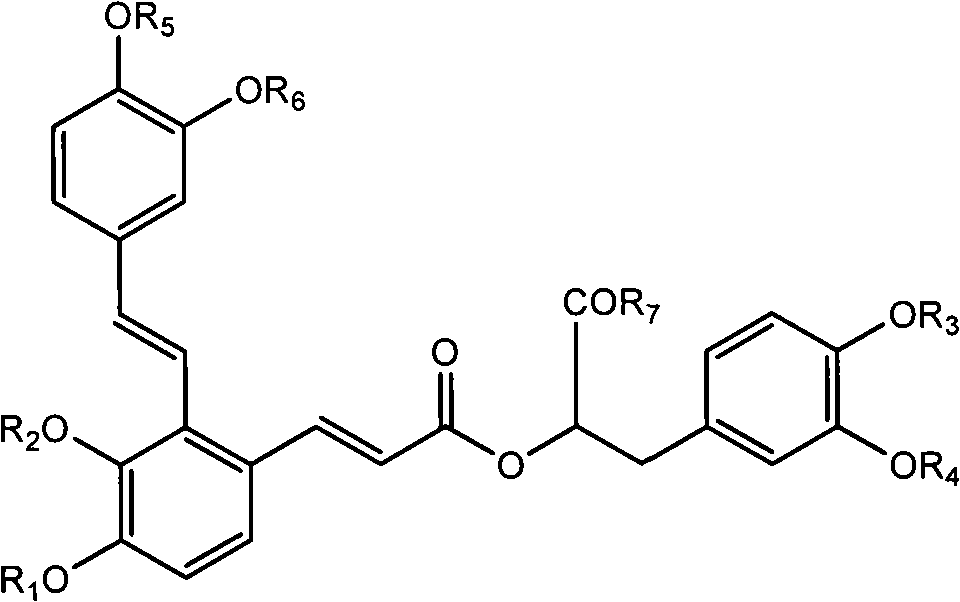
Compound and medicinal application thereof
A compound, drug technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0221] The raw material is: salvianolic acid A;
[0222] Preparation method: Take raw materials, add water to dissolve, add KOH solution, adjust pH value to 3-5, place at low temperature, crystallization occurs, filter, dry to obtain compound:
[0223]
[0224] Composing a pharmaceutical composition with the obtained compound as an active ingredient, or preparing it into a pharmaceutical preparation;
[0225] The obtained compound spectrum data are as follows:
[0226] Hydrogen spectrum (ppm): 6.83, 7.14, 7.82, 6.27, 6.69, 6.71, 6.82, 2.97, 2.82, 4.93, 7.03, 6.61, 6.44, 6.59, 7.03.
[0227] 碳谱(ppm):124.1、129.50、146.25、147.85、114.97、119.56、145.44、115.59、166.70、129.11、116.98、144.24、143.77、116.35、119.13、37.24、74.94、172.50、127.28、113.42、146.03、144.55、 115.90, 120.42, 135.94, 119.61.
Embodiment 2
[0229] The raw material is: salvianolic acid A;
[0230] Preparation method: add dichloromethane, raw materials, phenol, DCC and DMAP to a dry round bottom flask under nitrogen protection, a white flocculent precipitate is formed, stir at room temperature for 5 hours, TLC detects that the reaction is complete (developing agent: chloroform: methanol: formic acid =4:1:0.2). Suction filtration, wash the filtrate with dilute alkaline solution 3 times, then wash with saturated NaCl solution until neutral, dry over anhydrous sodium sulfate, filter, spin the filtrate, purify by column chromatography, collect the product phase, spin evaporate until dry to shallow Yellow foamy solid, yielding compound:
[0231]
[0232] Composing a pharmaceutical composition with the obtained compound as an active ingredient, or preparing it into a pharmaceutical preparation;
[0233] The obtained compound spectrum data are as follows:
[0234] Hydrogen spectrum (ppm): 6.86, 7.22, 8.05, 6.33, 6.7...
Embodiment 3
[0237] The raw material is: salvianolic acid A;
[0238] Preparation method: add the raw materials into dichloromethane under nitrogen protection, stir to dissolve, add triethylamine, drop the temperature to 0°C, add 1 propionic anhydride dropwise, after the dropwise addition, continue to react for 3 hours, TLC detects that the reaction is complete (developing agent: chloroform : methanol: formic acid=4:1:0.2). Add water dropwise to the reaction solution, separate the liquid, wash the organic phase with water, dry over anhydrous sodium sulfate, filter, spin the filtrate, purify by column chromatography, collect the product phase, spin evaporate to dryness to obtain a light yellow solid, and obtain the compound:
[0239]
[0240] Composing a pharmaceutical composition with the obtained compound as an active ingredient, or preparing it into a pharmaceutical preparation;
[0241] The obtained compound spectrum data are as follows:
[0242] Hydrogen spectrum (ppm): 7.24, 7.31...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com