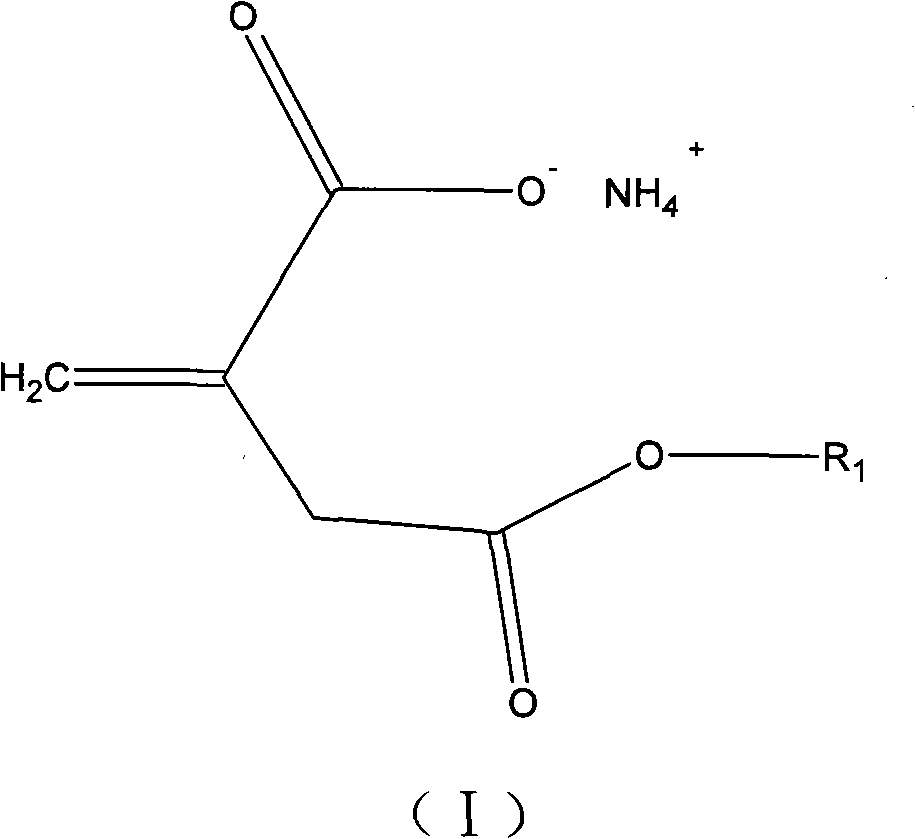
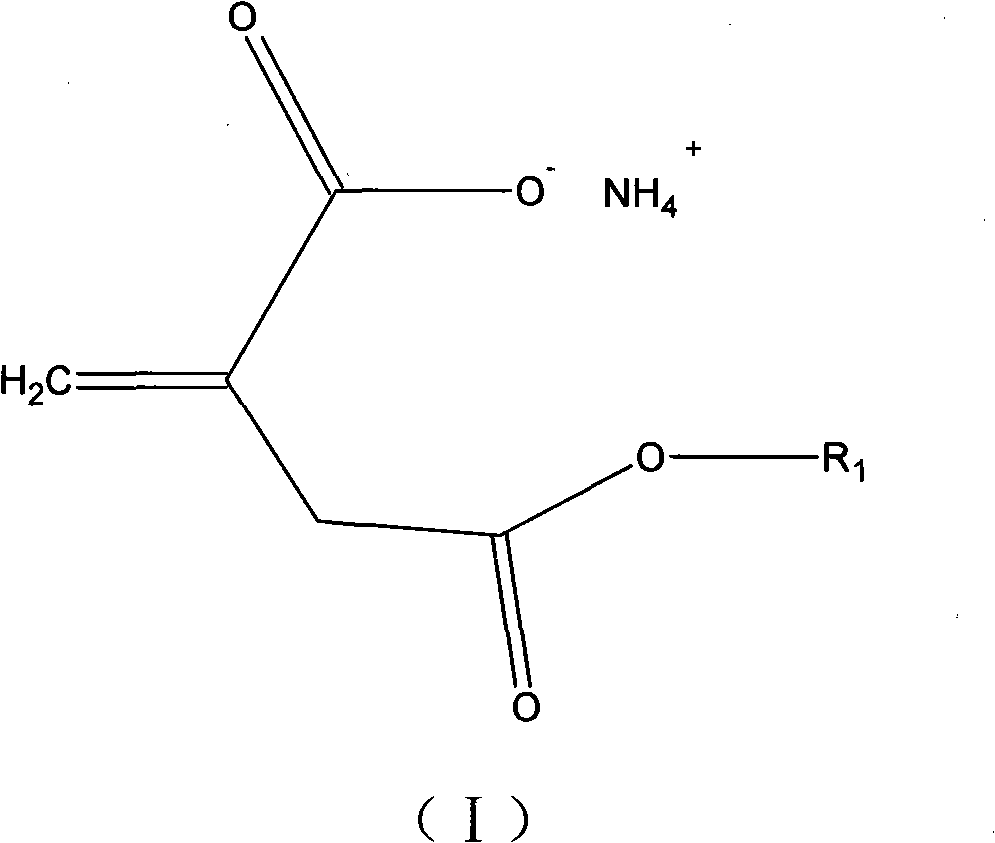
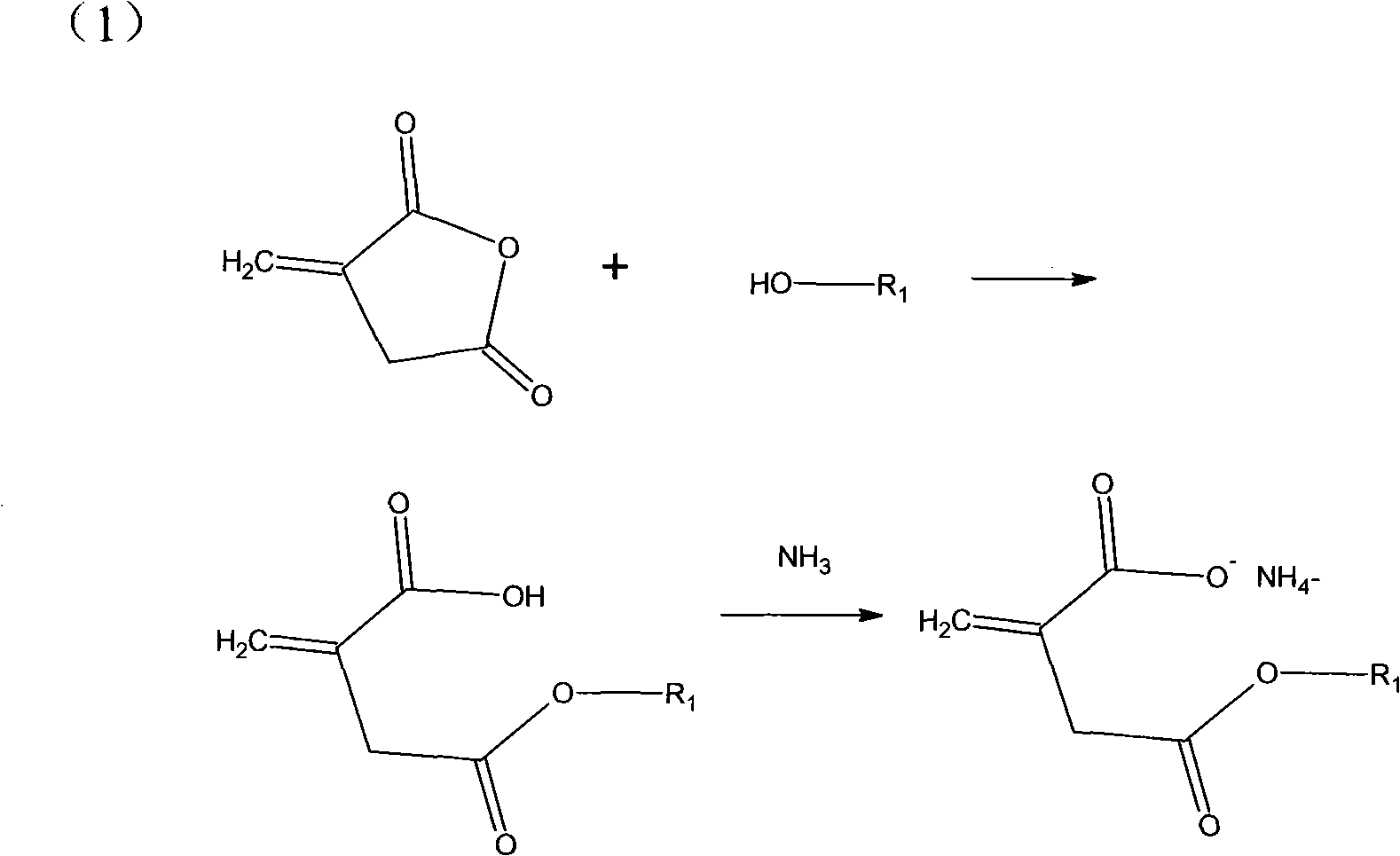
Itaconic acid derivant for copolymerization of acrylonitrile
A technology of methylene succinic acid and derivatives, which is applied in the field of methylene succinic acid derivatives, can solve the problems of difficult mass transfer and poor guarantee of polymer structure uniformity, and achieve controllability Improved effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Add 0.1 mol itaconic anhydride and 0.105 mol ethanol into a 50 ml conical flask, place it on a 30 degree constant temperature shaker for 24 hours, and then add 15 ml ammonia to the flask and shake for 30 minutes. The product was distilled under reduced pressure at 60 degrees for 2 hours to obtain a white solid, namely methylene succinic acid derivative, with a yield of 98%.
Embodiment 2
[0021] Add 0.1 mol itaconic acid, 0.2 mol methanol, and 0.1 ml concentrated hydrochloric acid to a 50 ml three-necked flask, stir the reaction at 50 degrees for 2 hours, titrate the acid value of the reactant to the theoretical value, and remove excess methanol and residual hydrochloric acid under reduced pressure. Add 15ml ammonia water and shake for 30min. The product was distilled under reduced pressure at 60 degrees for 2 hours to obtain a white solid, namely methylene succinic acid derivative, with a yield of 95%.
Embodiment 3
[0023] Put 30g acrylonitrile, 0.6g methylene succinic acid derivative (Example 1), and 120g dimethyl sulfoxide into a 250ml reaction flask, let the nitrogen flow for 30 minutes and then keep the temperature at 50 degrees, add 0.15g azobisisoheptonitrile After reacting for 12 hours under the protection of nitrogen, the obtained binary copolymer has a viscosity average molecular weight of 160,000, a molecular weight distribution of 2.2, and a monomer conversion rate of 91%.
[0024]
[0025] Acrylonitrile (R 1 Is an alkyl group with 2 carbon atoms)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com