Molecular design phycoerythrocyanin beta subunit fluorescent protein combining phycoerythrobilin and application thereof
A technology of phycoerythrocyanin and phycoerythrobilin, which is applied in the fields of application, algae/bryopeptides, hybrid peptides, etc., can solve the problems of complex technical procedures, high background, and difficult quantitative determination of fluorescence immunoassays, and achieve fluorescence High efficiency, good sensitivity, and easy purification effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
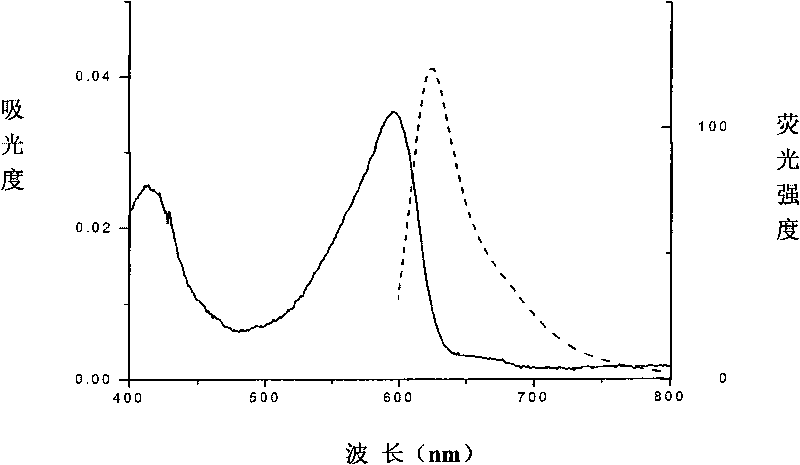
[0056] The amino acid sequence of the protein is shown in Sequence 1. The gene encoding the beta subunit of phycoerythrin was cloned into the expression plasmid, and the beta subunit of phycoerythrin was obtained by expression. Purification also helps to improve its solubility. Phycoerythrin is bound to the cysteine residue at position 201 (equivalent to position 153 of the beta subunit of original phycoerythrin) through a thioether bond. Its spectrum is figure 2 As shown, the absorption peak is at 547 nm and the fluorescence emission peak is at 566 nm.
Embodiment 2
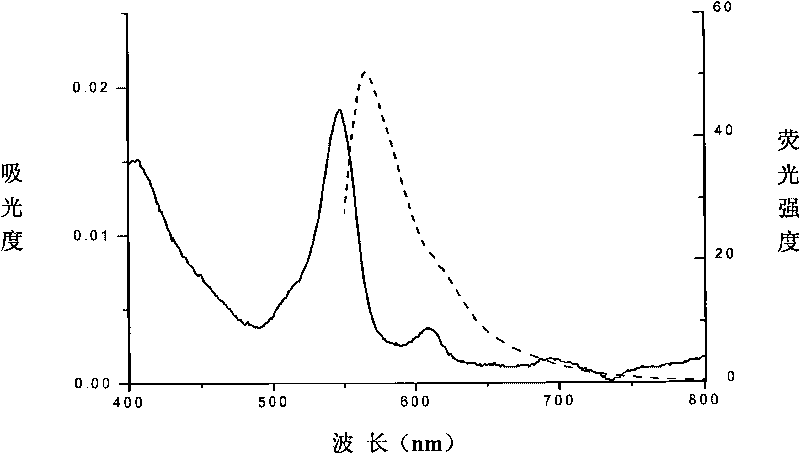
[0058] The amino acid sequence of the protein is shown in Sequence 2. The phycoerythrin beta subunit encoding gene is cloned into the expression plasmid, and it is mutated by genetic engineering methods to express the phycoerythrin beta subunit mutant, whose N-terminal band is Has a His-tag, which not only facilitates its purification, but also helps to improve its solubility. Phycoerythrin is bound to the cysteine residue at position 201 (equivalent to position 153 of the beta subunit of original phycoerythrin) through a thioether bond. Its spectrum is image 3 As shown, the absorption peak is at 547 nm and the fluorescence emission peak is at 566 nm.
Embodiment 3
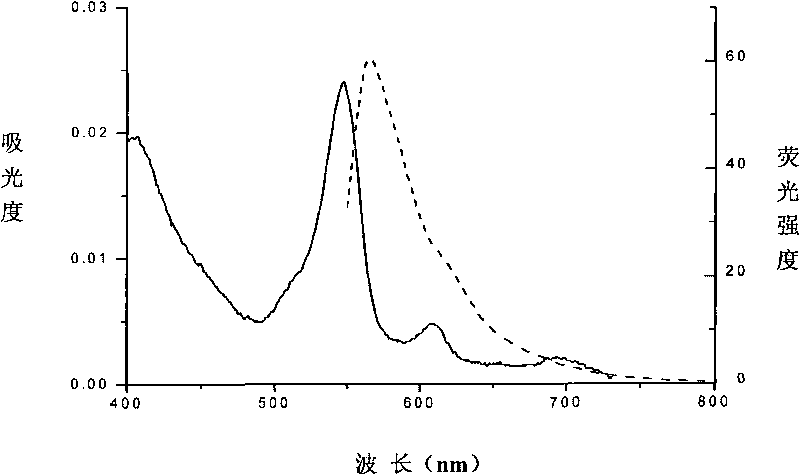
[0060] The amino acid sequence of the protein is shown in sequence 3. The gene encoding streptavidin and the beta subunit of phycoerythrin are spliced and cloned into the expression plasmid, and the obtained streptavidin and beta subunit of phycoerythrin are expressed. The fusion protein of phycoerythrin directly realizes the labeling of the beta subunit of phycoerythrin by streptavidin; and the N-terminus is tagged with His-tag, which is not only conducive to its purification, but also helps to improve its solubility. Phycoerythrin is bound to the cysteine residue at position 329 (equivalent to position 153 of the beta subunit of primitive phycoerythrin) through a thioether bond. Its spectrum is Figure 4 As shown, the absorption peak is at 547 nm and the fluorescence emission peak is at 566 nm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com