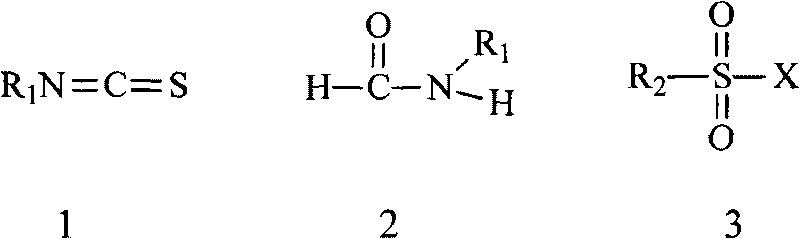
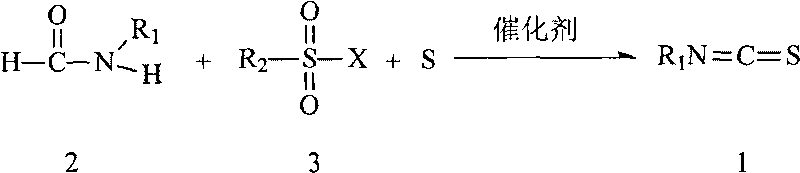Preparation method of isothiocyanate
A technology of elemental sulfur and compounds, applied in the direction of organic chemistry, can solve the problems of unsafe production, storage, transportation and use, great environmental hazards, and low yield of target substances, and achieve easy large-scale commercial preparation and simple preparation process , Environmentally friendly effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Synthesis of 1-naphthalene isothiocyanate:
[0031] Add 1-naphthylcarboxamide (0.5mmol), benzenesulfonyl chloride (0.5mmol), triethylamine (1.5mmol), sulfur (0.5mmol), and 5ml of polar solvent [alkane halide (such as CH 2 Cl 2 ) or alkyl substituted benzene, etc.], heated to reflux, and tracked the reaction with thin layer chromatography (TLC). The reaction was stopped when the raw material point disappeared, and the crude product was purified through a silica gel column with petroleum ether and ethyl acetate as eluents to obtain pure 1-naphthalene isothiocyanate with a melting point of 55.3°C. The yield was 86%.
Embodiment 2~12
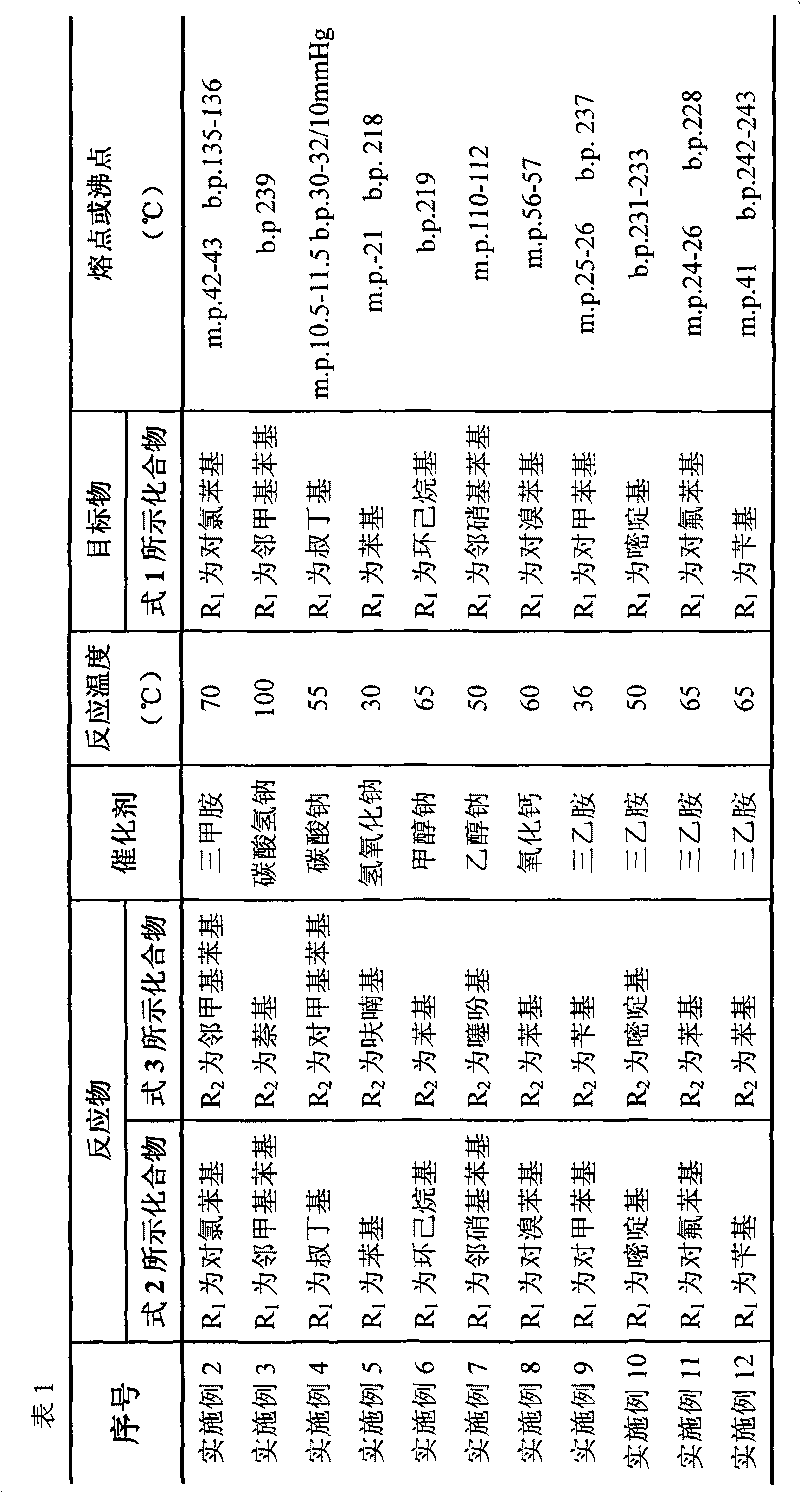
[0033] Using different raw materials (reactants), referring to the method of Example 1, different target objects can be obtained, see Table 1 for details.
[0034]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com