Drug composition of ligustrazine hydrochloride and benzyl alcohol and preparation method thereof
A technology of ligustrazine hydrochloride and its composition, which is applied in the pharmaceutical composition of ligustrazine hydrochloride and benzyl alcohol and its preparation field, can solve the problems of strong irritation and poor patient tolerance, and reduce irritation and vein irritation , the effect of improving tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
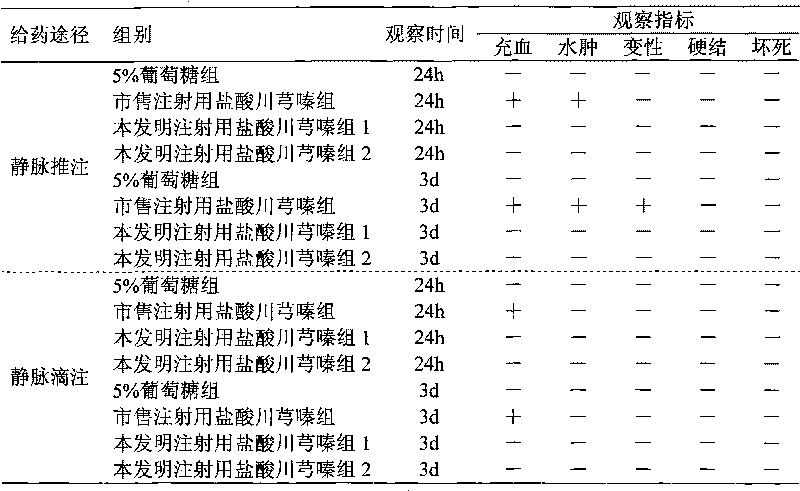
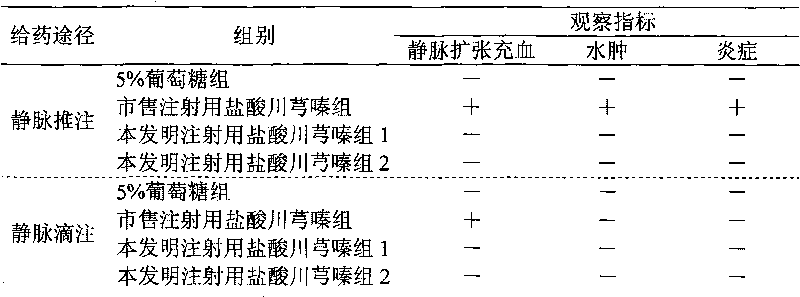
Image
Examples
Embodiment 1
[0038] 1. Prescription
[0039] prescription 1
[0040] Ligustrazine Hydrochloride 120g
[0041] Benzyl alcohol 5g
[0042] Mannitol 23g
[0044] Add water for injection to 2000mL
[0045]
[0046] A total of 1000 pieces were prepared
[0047] prescription 2
[0048] Ligustrazine hydrochloride 80g
[0049] Benzyl alcohol 1.5g
[0050] Mannitol 28.5g
[0052] Add water for injection to 2000mL
[0053]
[0054] A total of 1000 pieces were prepared
[0055] 2. Preparation method:
[0056] Weigh ligustrazine hydrochloride, benzyl alcohol, mannitol and sodium chloride according to the prescription, put them in a container, add 90% water for injection of the prescription, heat to 35°C, stir to dissolve, and add water to 2000mL;
[0057] Add 0.02% activated carbon for needles to the above solution, stir well at room temperature, filter to remove carbon, a...
Embodiment 2
[0060] 1. Prescription
[0061] prescription 1
[0062] Ligustrazine hydrochloride 80g
[0063]Benzyl alcohol 3g
[0064] Mannitol 137g
[0065] Add water for injection to 2000mL
[0066]
[0067] A total of 1000 sticks were prepared
[0068] Prescription 2
[0069] Ligustrazine Hydrochloride 120g
[0070] Benzyl alcohol 6g
[0071] Mannitol 204g
[0072] Add water for injection to 3000mL
[0073]
[0074] A total of 1000 sticks were prepared
[0075] 2. Preparation process:
[0076] Weigh ligustrazine hydrochloride, benzyl alcohol and mannitol according to the prescription, put them in a container, add 60% of the prescription amount of water for injection, heat to 30°C, stir to dissolve, add water to 3000mL;
[0077] Add 0.3% active carbon for needles to the above solution, stir well at room temperature, filter to remove carbon, and then fine filter with a 0.22 μm microporous membrane to measure the content...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com