Tirofiban hydrochloride lyophilized powder injection and preparation method thereof
A technology for tirofiban and injection, which is applied in the field of tirofiban hydrochloride freeze-dried powder injection and preparation, and can solve the problems of undisclosed pH value and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
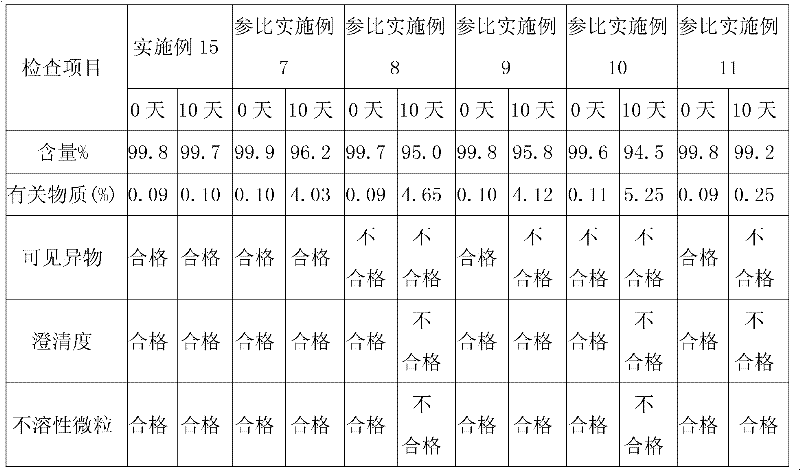
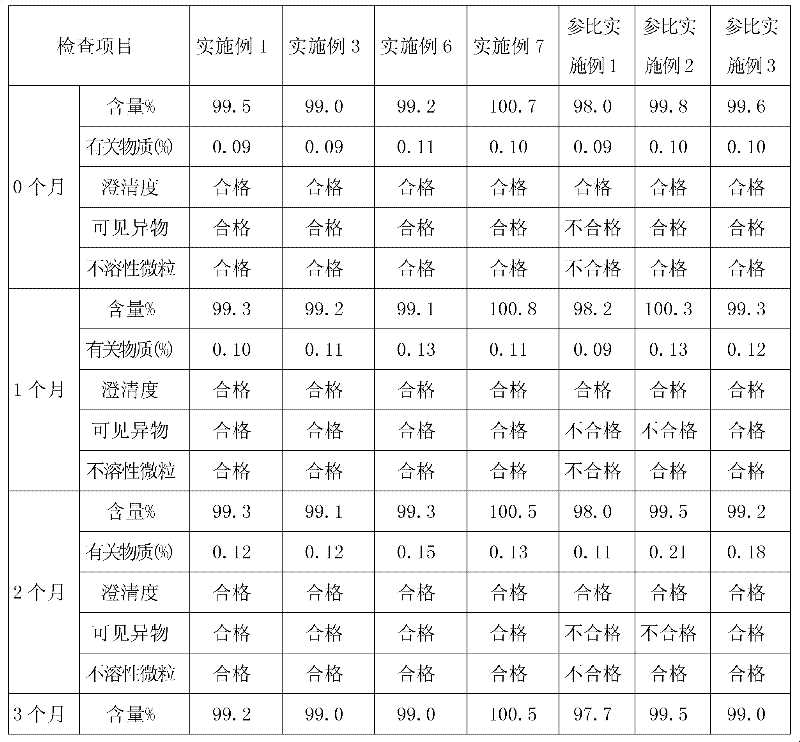
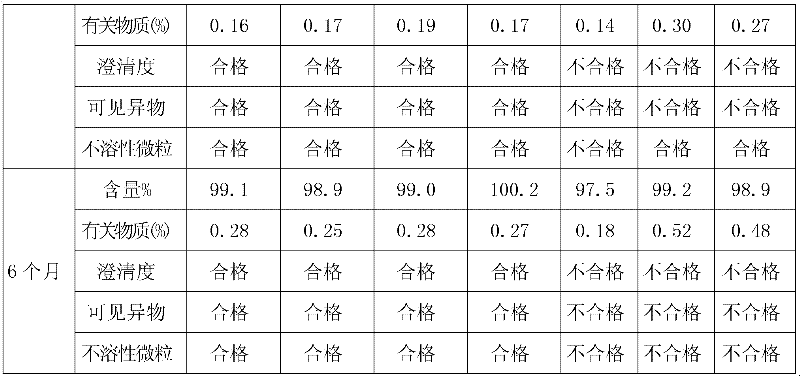
Examples
Embodiment 1
[0053] prescription
[0054] Tirofiban Hydrochloride 14.05g
[0055] Dextran-40 280.10g
[0056] Phosphoric acid appropriate amount
[0057] Make 1000 pieces
[0058] Preparation method: Weigh the prescribed amount of tirofiban hydrochloride and dextran-40, add an appropriate amount of water for injection to dissolve, adjust the pH value of the solution to 2.5 with phosphoric acid, sterilize and filter with a 0.22 μm microporous membrane, and quantitatively fill it in sterile clean pencillin Put in a bottle, half stoppered with rubber, freeze-dried, vacuum-pressed, rolled aluminum cap, and packaged.
Embodiment 2
[0060] prescription
[0061] Tirofiban Hydrochloride 14.05g
[0062] Dextran-407 0.25g
[0063] Citric acid amount
[0064] Make 1000 pieces
[0065] Preparation method: Weigh the prescribed amount of tirofiban hydrochloride and dextran-40, add an appropriate amount of water for injection to dissolve, adjust the pH value of the solution to 2.2 with citric acid, sterilize and filter with a 0.22 μm microporous membrane, quantitatively fill in sterile clean In vials, half stoppered with rubber, freeze-dried, vacuum-pressed, rolled aluminum caps, and packaged.
Embodiment 3
[0067] prescription
[0068] Tirofiban Hydrochloride 14.05g
[0069] Dextran-20 140.5g
[0070] Phosphoric acid appropriate amount
[0071] Make 1000 pieces
[0072] Preparation method: Weigh the prescribed amount of tirofiban hydrochloride and dextran-20, add an appropriate amount of water for injection to dissolve, adjust the pH value of the solution to 2.3 with phosphoric acid, sterilize and filter with a 0.22 μm microporous membrane, and quantitatively fill it in sterile clean pencillin Put in a bottle, half stoppered with rubber, freeze-dried, vacuum-pressed, rolled aluminum cap, and packaged.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com