Method for producing polymer membrane silver ion selective electrode
A silver ion, selective technology, applied in the direction of material analysis, measuring devices, instruments, etc. by electromagnetic means, can solve the problems of large amount of ion exchange resin, difficulty in miniaturization, short electrode life, etc., and achieve low detection limit, High selectivity, long life effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
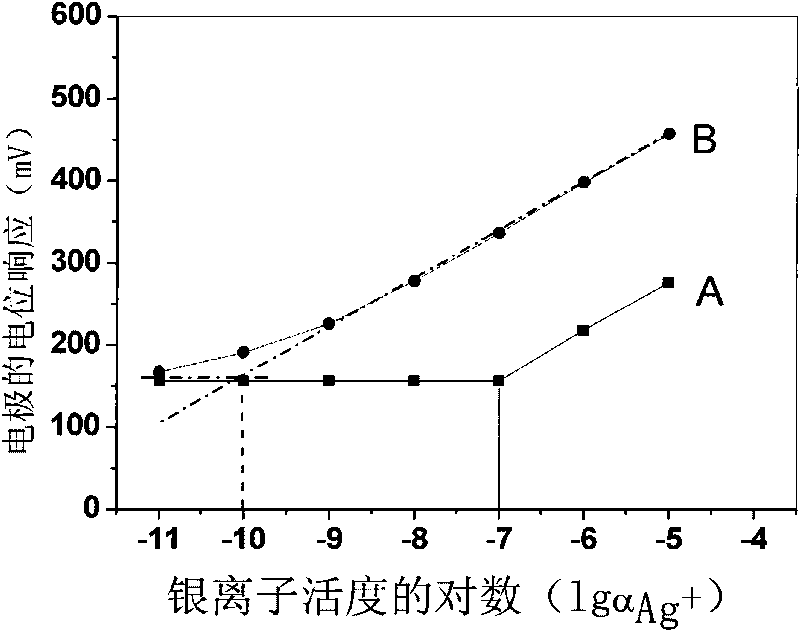
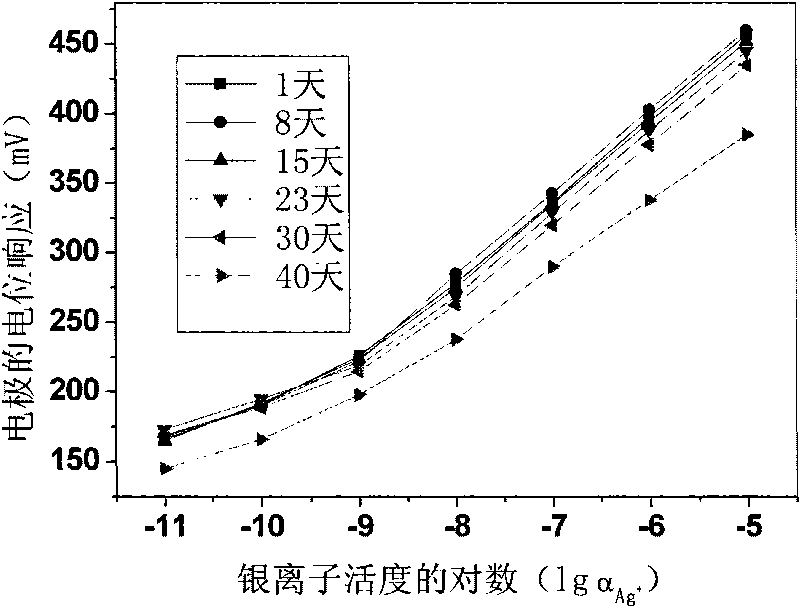
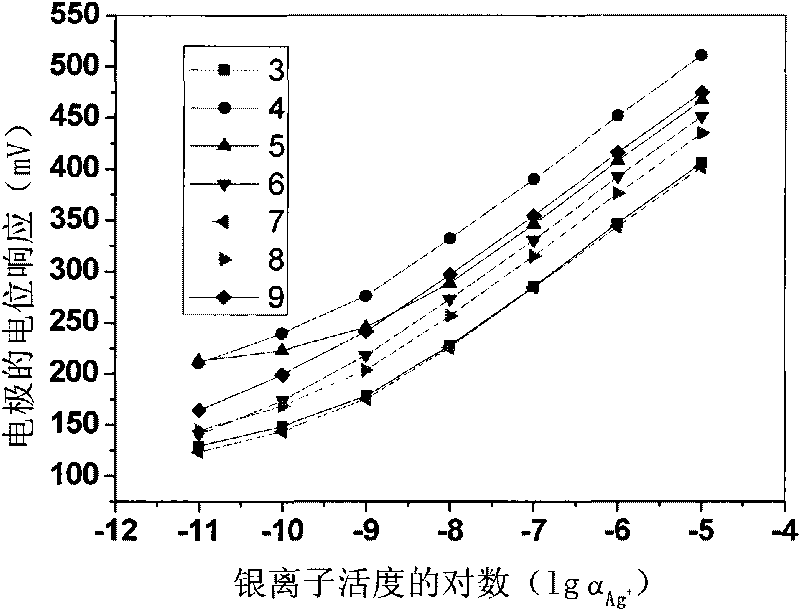
[0020] Weigh 0.17197g polyvinyl chloride, 0.31628g o-nitrophenyloctyl ether, 0.00247g tetrakis[3,5-bis(trifluoromethyl)phenyl]sodium borate, 0.00539g13,15,16, 18,20,26-Hexahydrotriphenyl[b,n,r][1,7,10,16,4,13]-tetrathiodiazaeicos-12,19(5H,11H) - Diketones are mixed, dissolved in tetrahydrofuran as a solvent, and stirred until transparent. The solution was poured on a horizontal glass plate to make a 0.25 mm film. Cut out a diaphragm with an inner diameter of 6 mm, and stick it to the end of the PVC plastic pipe with a PVC tetrahydrofuran solution. to 10 -2 M NaCl and 10 -5 MAgNO 3 For inner filling, use 10 -5 M silver nitrate solution was activated for 48 hours to prepare a silver ion polymer membrane electrode with a low detection limit, and the silver wire was used as an internal reference electrode to measure silver ions. Electrodes at 10 -9 M-10 -5 Nernst response in the M range.
Embodiment 2
[0022] Weigh 0.17192g polyvinyl chloride, 0.31621g dioctyl phthalate, 0.00249g tetrakis[3,5-bis(trifluoromethyl)phenyl]sodium borate, 0.00535g13,15,16 in a weighing bottle , 18,20,26-hexahydrotriphenyl[b,n,r][1,7,10,16,4,13]-tetrathiodiazaeicos-12,19(5H,11H )-diketone mixed, dissolved in tetrahydrofuran as a solvent, and stirred until transparent. The solution was poured onto a horizontal glass plate to produce a 0.26mm film. Cut out a diaphragm with an inner diameter of 6 mm, and stick it to the end of the PVC plastic pipe with a PVC tetrahydrofuran solution. to 10 -2 M NaCl and 10 -5 M AgNO 3 For the inner filling liquid, use 10 -5 M silver nitrate solution was activated for 48 hours to prepare a silver ion polymer membrane electrode with a low detection limit, and the silver wire was used as an internal reference electrode to measure silver ions.
Embodiment 3
[0024] Weigh 0.34605g polyvinyl chloride, 0.17962g dioctyl phthalate, 0.00247g tetrakis[3,5-bis(trifluoromethyl)phenyl]sodium borate, 0.00539g13,15,16 in a weighing bottle , 18,20,26-hexahydrotriphenyl[b,n,r][1,7,10,16,4,13]-tetrathiodiazaeicos-12,19(5H,11H )-diketone mixed, dissolved in tetrahydrofuran as a solvent, and stirred until transparent. The solution was poured onto a horizontal glass plate to produce a 0.26mm film. Cut out a diaphragm with an inner diameter of 6 mm, and stick it to the end of the PVC plastic pipe with a PVC tetrahydrofuran solution. with 1M NaCl and 10 -5 M AgNO 3 For the inner filling liquid, use 10 -5 M silver nitrate solution was activated for 48 hours to prepare a silver ion polymer membrane electrode with a low detection limit, and the silver wire was used as an internal reference electrode to measure silver ions.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com