Application of pinocembrin raceme in preparation of medicals for cerebral apoplexy
A technology of raceme and pinogenin, which is applied in the field of preparation of the prevention and treatment of stroke drugs by the raceme of pinogenin, can solve the problems of lack of effective treatment of stroke and application restrictions, and achieve the protection of nerve cell damage , improve behavioral changes, and improve the effect of cerebral edema
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
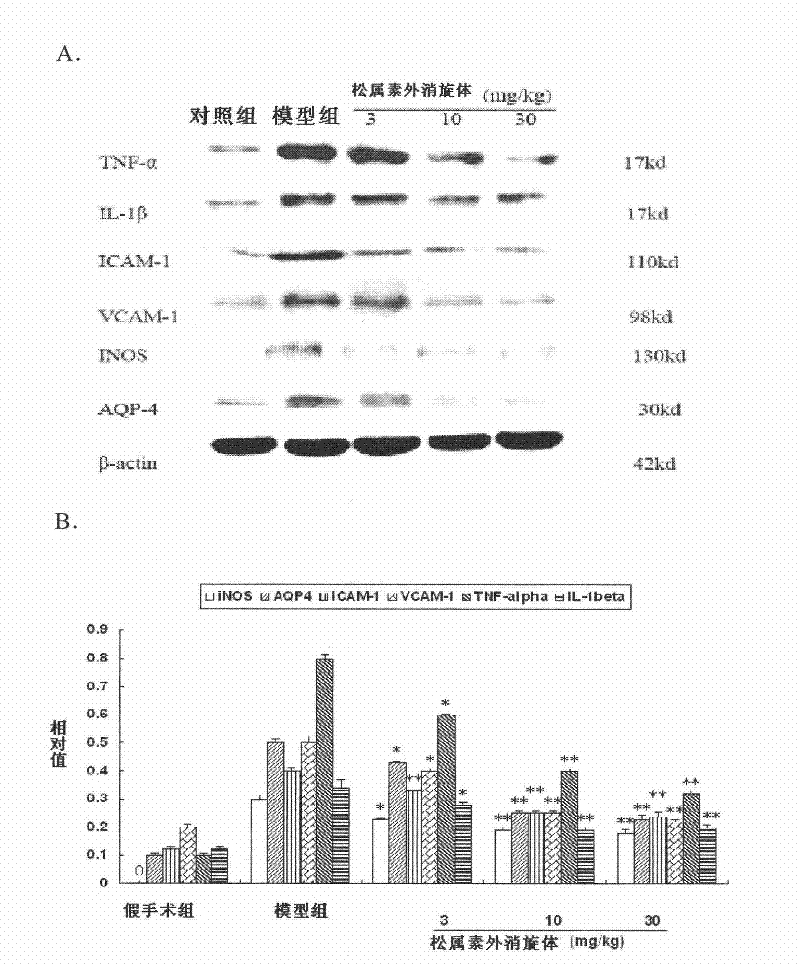
[0060] Example 1. Preventive and therapeutic effects on apoplexy in stroke-prone spontaneously hypertensive rats (SHRSP)
[0061] 1. Experimental drugs: pinocin racemate for injection, (R)-pinocin for injection and (S)-pinocin for injection, provided by the New Drug Development Office of the Institute of Materia Medica, Chinese Academy of Medical Sciences, (batch number 20050601, Content 2.36%), adopt the method disclosed in 200810084682.3 that is cyclodextrin or cyclodextrin derivatives inclusion racemization and isomer pinazin to prepare, and dissolve in normal saline when used; positive drug nimodipine injection is purchased from Bayer company (Germany).
[0062] 2. Experimental animals and groups:
[0063] Test animals: 110 6-week-old SHRSP rats and 10 normal Wistar rats.
[0064] Test grouping: be divided into normal group namely Wistar rat group, SHRSP rat is divided into model group, nimodipine group, pinocin racemate low, middle and high dose groups at random (dose i...
Embodiment 2
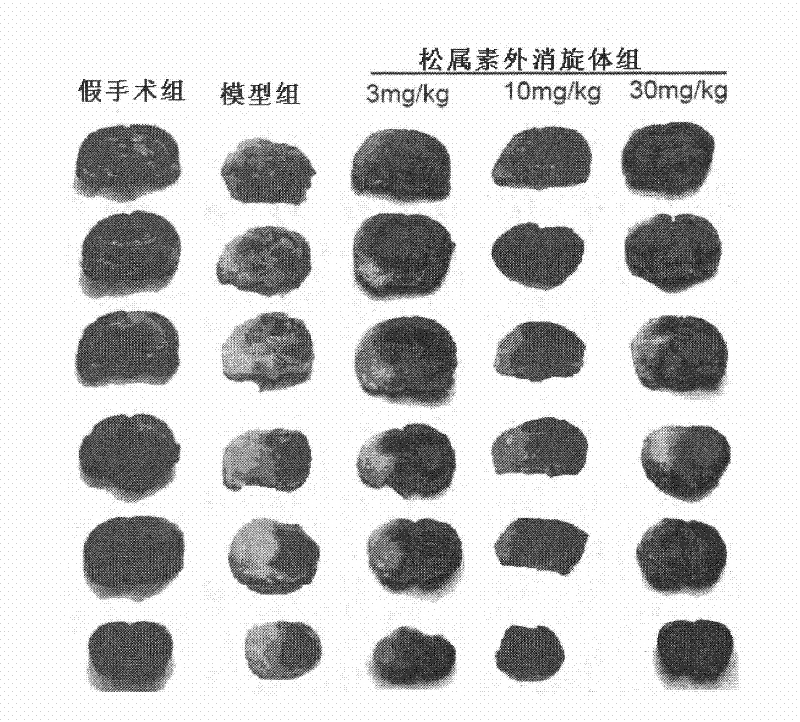
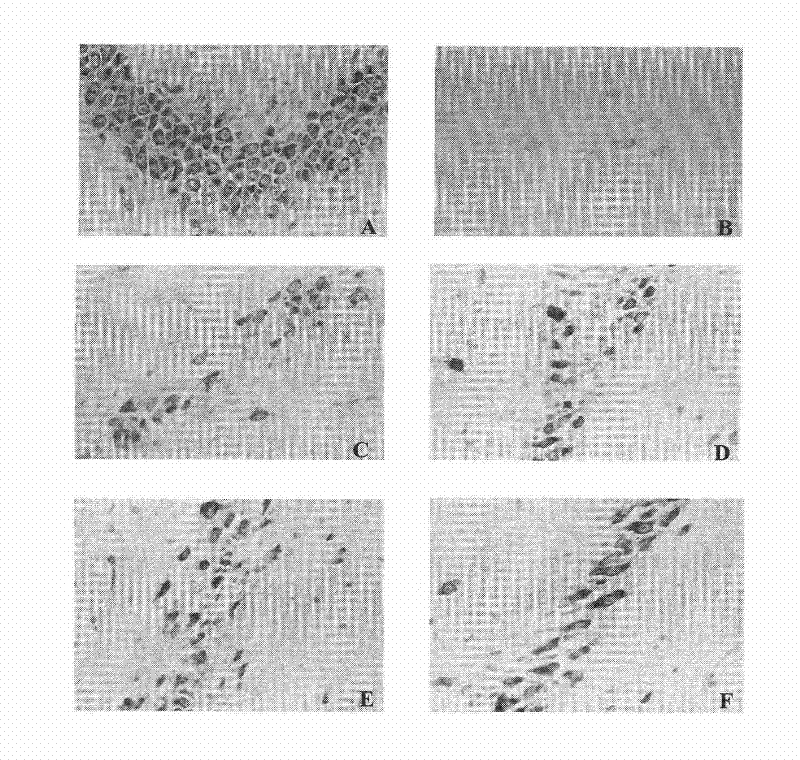
[0123] Example 2. Effect on acute ischemic stroke caused by middle cerebral artery embolism
[0124] Test substance: pinoquinone for injection, provided by the New Drug Development Office of the Institute of Materia Medica, Chinese Academy of Medical Sciences,
[0125] (batch number 20050601, content 2.36%), it is prepared by adopting the method disclosed in 200810084682.3 that cyclodextrin or cyclodextrin derivatives include pinocin racemate, and is dissolved in normal saline when used; the positive drug nimodipine injection is purchased at Bayer AG (Germany).
[0126] Experimental animals: 100 male SD rats, weighing 250-280 grams, were purchased from the Institute of Experimental Animals, Chinese Academy of Medical Sciences. The rats were randomly divided into: sham operation group, model group, pinecone administration group, and nimodipine administration group (3mg / kg).
[0127] Experimental model: using middle cerebral artery occlusion (MCAO) to prepare acute ischemic st...
Embodiment 3
[0198] Embodiment three, acute toxicity study result and evaluation
[0199] The test results show that the LD50 value of SD rats' single intravenous injection of pinecine racemate toxic reaction is 490.9 (367.6-746.7) mg / kg, and the LD50 value of (S)-pinecin toxic reaction is 375.3 (271.2 -538.5) mg / kg, the LD50 value of (R)-pinine toxicity is 347.8 (257.4-466.3) mg / kg. It can be seen from the above that the racemic form of pinocin has a relatively large range of safe drug use. All the drugs had no obvious effect on the body weight of the animals, and the main toxic manifestations were quadriplegia, mild congestion of the liver and lungs.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com