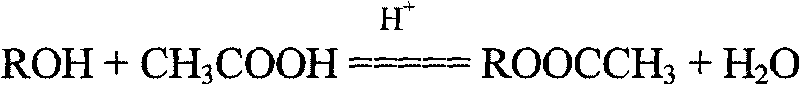Esterification and termination synthesis method of allyl polyether
A technology of allyl polyether and synthesis method, which is applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., and can solve problems such as high cost, little practical significance, and environmental pollution by sodium chloride
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Add 414g allyl polyoxyethylene ether (hydroxyl value 135.5mgKOH / g), 1.53g p-toluenesulfonic acid, 153g toluene, 96g acetic acid into a 1L four-neck flask equipped with a mechanical stirrer, reflux condenser and water trap. , Turn on the condensed water, turn on the stirring device, and heat to make the reaction proceed. When the temperature is raised to boiling, the time is started, and the water separation operation 5h ends. Then, when the temperature drops to normal temperature, the reaction device is switched to a vacuum distillation device to remove toluene and unreacted acetic acid, and then undergo a refining process to obtain a colorless esterified end-capped polyether product. The measured hydroxyl value of the product was 3.7 mgKOH / g, and the esterification end-capping rate was 97.3%.
Embodiment 2
[0038] Add 414g allyl polyoxyethylene ether (hydroxyl value 135.5mgKOH / g), 2.04g p-toluenesulfonic acid, 204g toluene, 96g acetic acid into a 1L four-neck flask equipped with a mechanical stirrer, reflux condenser and water trap. , Turn on the cooling water, turn on the stirrer, heat up to boiling and start timing, and the water splitting operation ends for 7 hours. Then, when the temperature drops to normal temperature, the reaction device is switched to a vacuum distillation device to remove toluene and unreacted acetic acid, and then undergo a refining process to obtain a colorless esterified end-capped polyether product. The measured hydroxyl value of the product was 2.2 mgKOH / g, and the esterification capping rate was 98.4%.
Embodiment 3
[0040] Add 414g allyl polyoxyethylene ether (hydroxyl value 135.5mgKOH / g), 1.05g p-toluenesulfonic acid, 209g toluene, 108g acetic acid into a 1L four-neck flask equipped with a mechanical stirrer, reflux condenser and water trap. , Turn on the cooling water, turn on the stirrer, heat up to boiling and start timing, and the water splitting operation ends for 3 hours. Then, when the temperature drops to normal temperature, the reaction device is switched to a vacuum distillation device to remove toluene and unreacted acetic acid, and then undergo a refining process to obtain a colorless esterified end-capped polyether product. The measured hydroxyl value of the product is 11.5 mgKOH / g, and the esterification end-capping rate is 91.5%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hydroxyl value | aaaaa | aaaaa |
| Hydroxyl value | aaaaa | aaaaa |
| Hydroxyl value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com