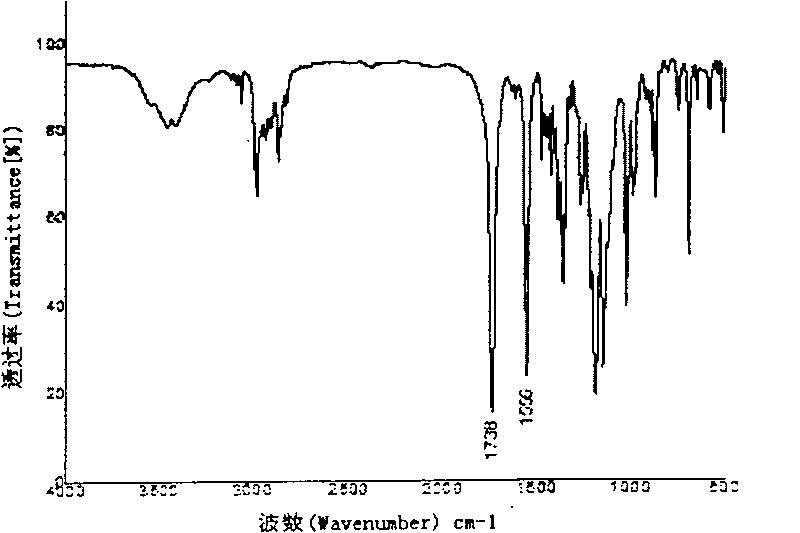
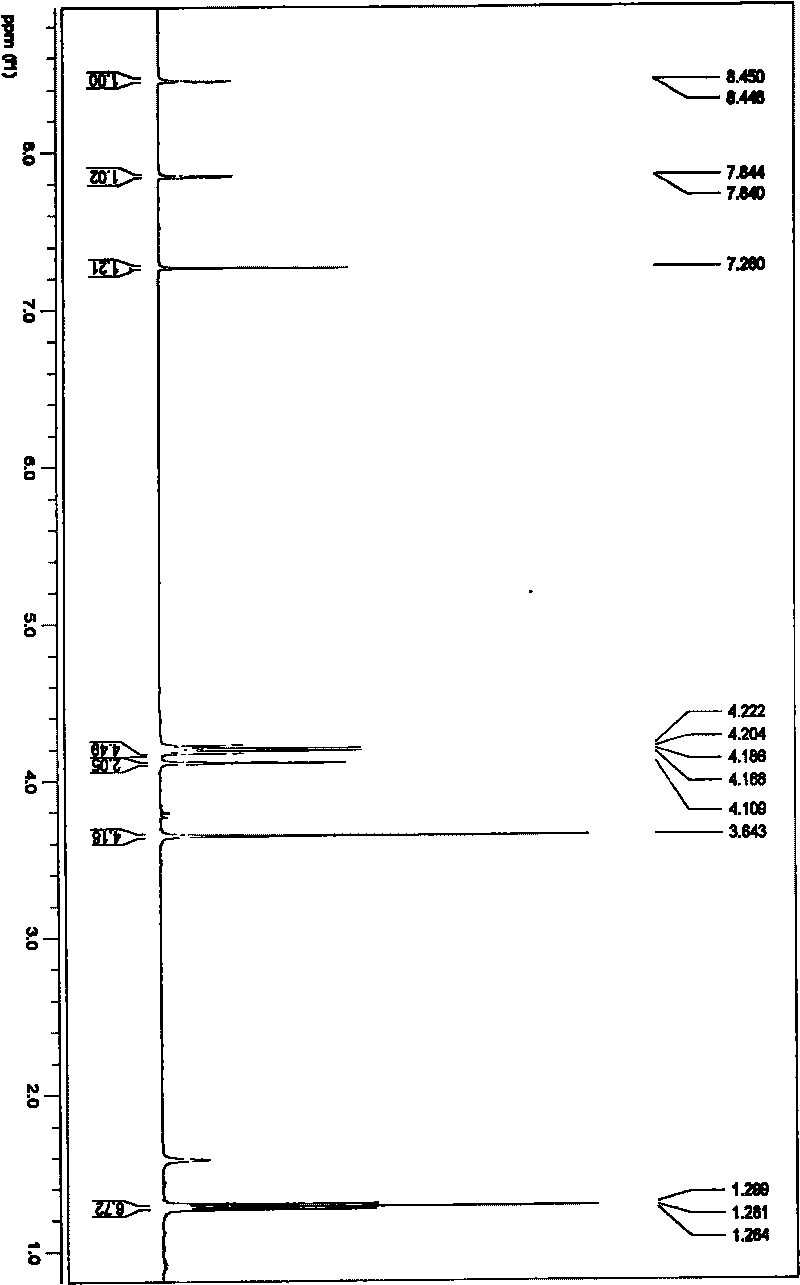
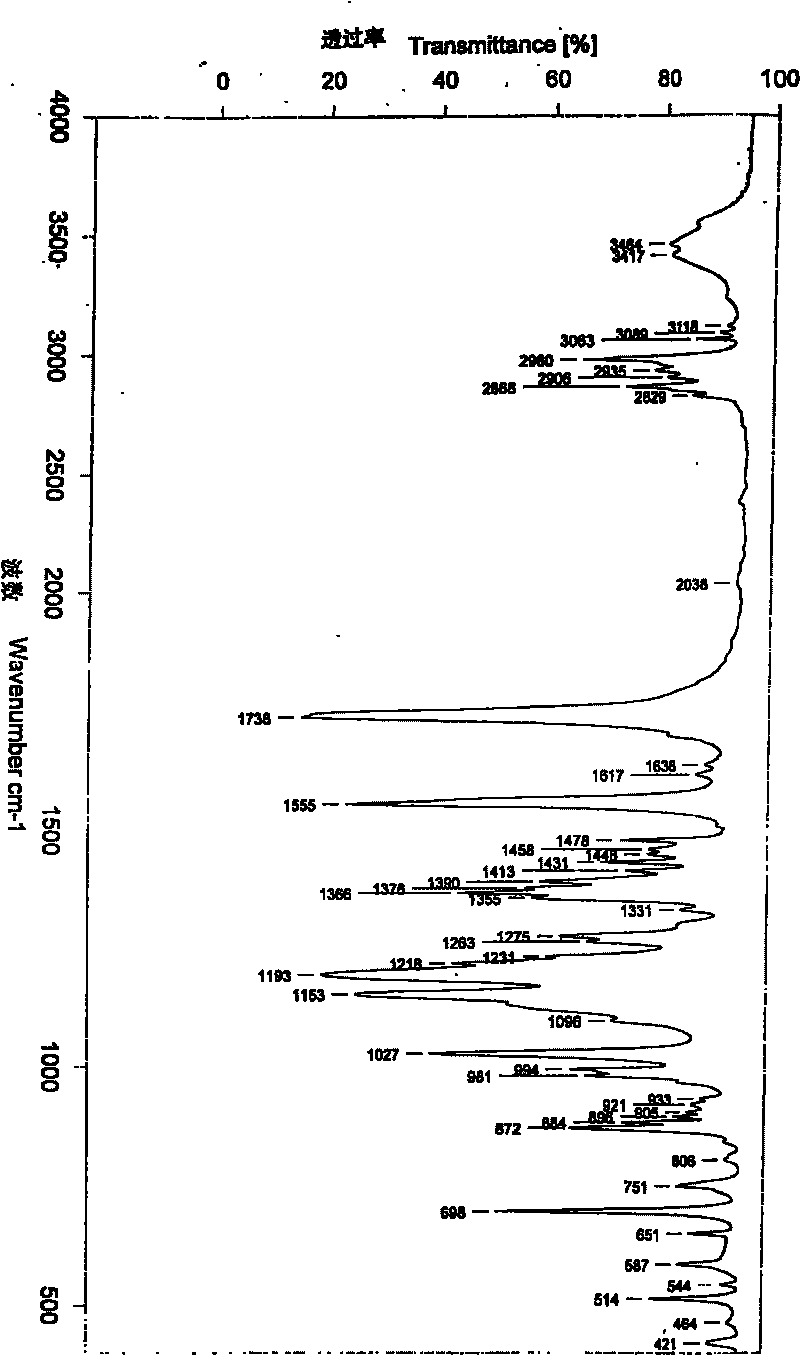
Rare earth europium fluorescent chelating agent and preparation method thereof
A chelating agent and fluorescent technology, applied in chemical instruments and methods, luminescent materials, organic chemistry, etc., can solve problems such as poor optical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0101] (1) Preparation of 2-bromo-6-picoline
[0102] Dissolve 70g of 2-amino-6-picoline in 330ml of HBr solution with a mass concentration of 40%, keep it at 0°C, and add 100ml of Br with a concentration of 0.02mol / ml dropwise 2 , then dropwise add 175ml of aqueous solution containing sodium nitrite 0.66g / ml, stir for 30min, continue to dropwise add 265ml of aqueous solution containing NaOH 0.95g / ml, stir for another 30min, extract with dichloromethane, and use MgSO 4 After absorbing water and drying, vacuum distillation collected 103 g of distillate 2-bromo-6-picoline at 4 mmHg at 62 ° C, bp 129-130 ° C, yield 72%.
[0103] (2) add 2-bromo-6-picoline 88g in the reactor, sodium formate dihydrate 82.0g, be the palladium carbon catalyst 3.2g of 5% with mass concentration, benzyltriethylammonium chloride 21.2g (0.08 mol), mass concentration is 32% NaOH solution 53ml, H 2 O 130ml, stirred, heated to reflux reaction 48h, added 3.8g sodium formate dihydrate, 0.8g palladium carbon...
Embodiment 2
[0122] (1) Preparation of 2-bromo-6-picoline
[0123] Dissolve 73g of 2-amino-6-picoline in 330ml of HBr solution with a mass concentration of 40%, keep it at -8°C, and add 100ml of Br with a concentration of 0.02mol / ml dropwise 2 , then dropwise add 175ml of aqueous solution containing sodium nitrite 0.66g / ml, stir for 30min, continue to dropwise add 265ml of aqueous solution containing NaOH 0.95g / ml, stir for another 30min, extract with dichloromethane, and use MgSO 4 After absorbing water and drying, vacuum distillation collected 103 g of distillate 2-bromo-6-methylpyridine at 4 mmHg at 63°C, bp 129-130°C, yield 71%.
[0124] (2) Preparation of 6,6'-dimethyl-2,2'-bipyridine
[0125] Add 2-bromo-6-picoline 90g in reactor, sodium formate dihydrate 82.0g, be 5% palladium carbon catalyst 3.2g, benzyltriethylammonium chloride 21.2g (0.08mol) with mass concentration, Mass concentration is 32% NaOH solution 53ml, H 2 O 130ml, stirred, heated to reflux reaction for 48h, added ~5...
Embodiment 3
[0140] (1) Preparation of 2-bromo-6-picoline
[0141] Dissolve 75g of 2-amino-6-picoline in 330ml of HBr solution with a mass concentration of 40%, keep it at -15°C, and add 100ml of Br with a concentration of 0.02mol / ml dropwise2 , then dropwise add 175ml of aqueous solution containing sodium nitrite 0.66g / ml, stir for 30min, continue to dropwise add 265ml of aqueous solution containing NaOH 0.95g / ml, stir for another 30min, extract with dichloromethane, and use MgSO 4 After absorbing water and drying, vacuum distillation collected 103 g of distillate 2-bromo-6-methylpyridine at 4 mmHg at 65 °C, bp129-130 °C, yield 70%.
[0142] (2) Preparation of 6,6'-dimethyl-2,2'-bipyridine
[0143] Add 2-bromo-6-picoline 92g in reactor, sodium formate dihydrate 82.0g, be 5% palladium carbon catalyst 3.2g, benzyltriethylammonium chloride 21.2g (0.08mol) with mass concentration, Mass concentration is 32% NaOH solution 53ml, H 2 O 130ml, stirred, heated to reflux reaction 48h, added 11.5g ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com