Beta-lactam twin antibiotic compound, preparation method thereof and use thereof
A technology of lactams and compounds, applied in the field of medicine, can solve the problems of bacterial resistance and cross-resistance, and achieve significant antibacterial activity and the effect of overcoming drug resistance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
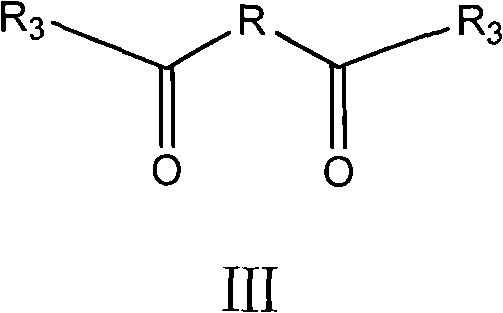
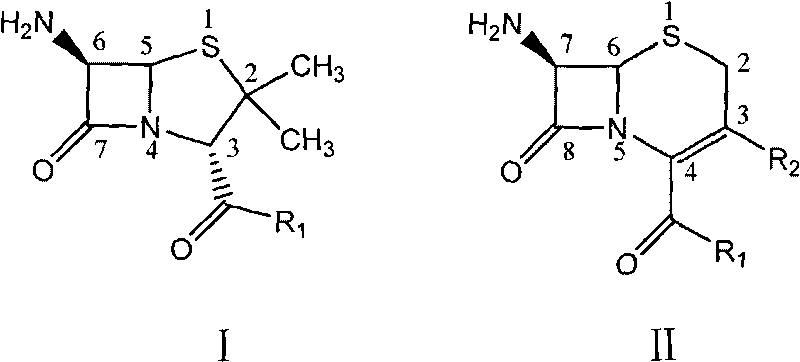
[0068] Preparation of N,N'-bis-[(2S,5R,6R)-3,3-dimethyl-7-oxo-4-thio-1-azabicyclo[3,2,0]heptane-2 -Carboxylic acid-6-yl]-1,6-hexanediamide
[0069] In a dry 250ml three-neck flask, add 0.73g (5.0mmol) of adipic acid and 50ml of tetrahydrofuran, cool to -5°C in an ice-salt bath, and then add 2.4g of N,N-dicyclohexylcarbodiimide (DCC) (12.0mmol) and N-hydroxysuccinimide (NHS) 1.4g (10.0mmol), naturally heated and stirred for 6h, filtered to remove solid N,N-dicyclohexyl urea, the filtrate was decompressed to recover the solvent, washed with a small amount of tetrahydrofuran The N-hydroxysuccinimide ester of 1,6-adipic acid is obtained. Add 15ml of N,N-dimethylformamide to another reaction flask, cool down to 5-10°C in an ice bath, add 2.16g (10.0mmol) of 6-APA and 1.5ml of triethylamine, and stir for 15 minutes. The above ester was then dissolved in a small amount of N,N-dimethylformamide and added to the reaction flask, and stirred overnight. Add 50 ml of ice water, adjust t...
Embodiment 2
[0072] Preparation of N,N'-bis-[(6R,7R)-3-(acetoxymethyl)-8-oxo-5-thio-1-azabicyclo[4,2,0]oct-2-ene -2-Carboxylic acid-7-yl]-1,6-hexanediamide
[0073] In a dry 100ml three-necked flask, add 0.73g (5.0mmol) of adipic acid, 15ml of benzene and 5 drops of N,N-dimethylformamide, heat to 60-70°C, add dropwise 1.2g (4.0mmol) The benzene solution of triphosgene (bistrichloromethanol carbonate) was dropped in about 30 minutes. Continue to stir for 4 hours after the addition, transfer to a 100ml round bottom bottle, recover the solvent, and obtain a light yellow adipoyl chloride liquid, which is stored at low temperature until use.
[0074] In a 250ml three-necked flask, add 2.72g (10.0mmol) of 7-ACA, 20ml of water, 0.5g of disodium hydrogen phosphate and 1.5ml of triethylamine, and slowly add 5% sodium hydroxide solution dropwise until the solution is clear. Then add 15ml of water, 15ml of acetone, a little tetrabutylammonium bromide, adjust the pH to 8-9 with saturated sodium bica...
Embodiment 3
[0077] Preparation of N,N'-bis-[(6R,7R)-8-oxo-5-thio-1-azabicyclo[4,2,0]oct-2-ene-2-carboxylic acid-7-yl ]-1,6-Hipamide
[0078] In a dry 250ml three-necked flask, add 0.73g (5.0mmol) of adipic acid and 50ml of tetrahydrofuran, cool to about -5°C in an ice-salt bath, and then add 2.4g (12.0 mmol) and N-hydroxysuccinimide (NHS) 1.4g (10.0mmol), naturally heated and stirred for 6h, filtered to remove solid N, N-dicyclohexyl urea, the filtrate recovered solvent, washed with a small amount of tetrahydrofuran to obtain 1,6 -N-Hydroxysuccinimide ester of adipic acid. Add 15ml of N,N-dimethylformamide to another reaction flask, cool down to 0-5°C in an ice bath, add 2.0g (10.0mmol) of 7-ANCA and 1.5ml of triethylamine, stir for 15 minutes, and use A small amount of N,N-dimethylformamide was added to the reaction flask after dissolving the above-mentioned N-hydroxysuccinimide ester of 1,6-adipic acid, and stirred overnight. Add 50ml of ice water, adjust the pH to 1-2 with 1N hydroc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com