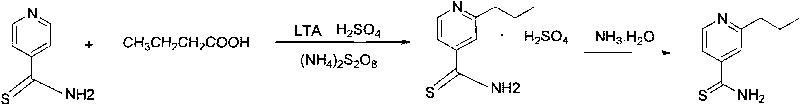Preparation method of 2-alkyl thioisonicotinamide
A technology of alkyl thioisonicotinamide and thioisonicotinamide is applied in the direction of organic chemistry, etc., can solve the problems of expensive catalyst silver nitrate, low selectivity of alkylation reaction, no practical value, etc., and achieves improved reaction The effect of high conversion, high selectivity, and easy to scale production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0020] The preparation method of 2-alkylthioisonicotinamide of the present invention uses 4-cyanopyridine as a raw material, and 4-cyanopyridine and ammonium sulfide react under the catalysis of sulfur to obtain thioisonicotinamide; Niacinamide and fatty acid undergo an alkylation reaction under the catalysis of lead tetraacetate (LTA) to obtain the crude product of 2-alkylthioisonicotinamide, which is refined with ethanol to obtain the fine product. The selectivity of the alkylation reaction can be effectively improved, the purity of the product can be significantly improved, and the step of rectification is not required, so it is better suitable for industrial application.
[0021] The specific process is: first, add sulfur to 20% ammonium sulfide aqueous solution, heat and stir until dissolved, then add 4-cyanopyridine to react, wherein the molar ratio of 4-cyanopyridine to ammonium sulfide and sulfur is 1 : (2~4): (0.3~1), the temperature of reaction is 40~60 ℃, and the ti...
Embodiment 1
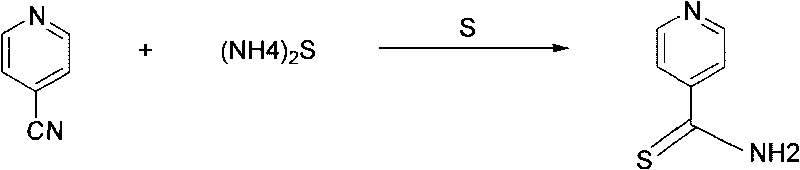
[0023] Synthesis of thioisonicotinamide: Put 294g of 20% ammonium sulfide aqueous solution into a four-neck flask, add 4.2g of sulfur under stirring, heat and stir until completely dissolved, add 45g of 4-cyanopyridine, heat up to 40°C, keep warm 4 hours; the reaction formula is as follows figure 1 shown.
[0024] After the reaction, the temperature was lowered to 25°C, suction filtered, and the filter cake was washed with distilled water at 70°C until neutral to obtain thioisonicotinamide, which was dried to obtain 47.3g.
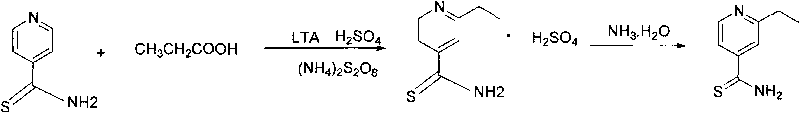
[0025] Synthesis of 2-ethylthioisonicotinamide: 56.8g ammonium persulfate was dissolved in 113.6g water to make ammonium persulfate solution, set aside. Add 47.3g of thioisonicotinamide, 23.6g of concentrated sulfuric acid, and 1.42g of lead tetraacetate to 240g of water, add 50.7g of propionic acid and ammonium persulfate solution under stirring, and react at 60°C for 2 hours; the reaction formula is as follows: figure 2 shown.
[0026] After the reac...
Embodiment 2
[0028] Synthesis of thioisonicotinamide: Put 441.6g of 20% ammonium sulfide aqueous solution into a four-neck flask, add 6.9g of sulfur under stirring, heat and stir until completely dissolved, add 45g of 4-cyanopyridine, heat up to 50°C, Keep warm for 5 hours; after the reaction, cool down to 25°C, filter with suction, wash the filter cake with distilled water at 70°C until neutral to obtain thioisonicotinamide, and dry to obtain 49.8g.
[0029] Synthesis of 2-propylthioisonicotinamide: 74.7g of ammonium persulfate was dissolved in 149.4g of water to make an ammonium persulfate solution for subsequent use. Add 49.8g of thioisonicotinamide, 29.9g of concentrated sulfuric acid, and 1.6g of lead tetraacetate to 350g of water, add 63.5g of butyric acid and ammonium persulfate solution under stirring, and react at 70°C for 2 hours; the reaction formula is as follows: image 3 shown.
[0030] After the reaction, the temperature was lowered to 25°C, ammonia water was slowly added t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com