Thymopentin oral microsphere preparation and preparation method thereof
A technology of microspheres and preparations, applied in pharmaceutical formulations, peptide/protein components, medical preparations containing active ingredients, etc. The effect of bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
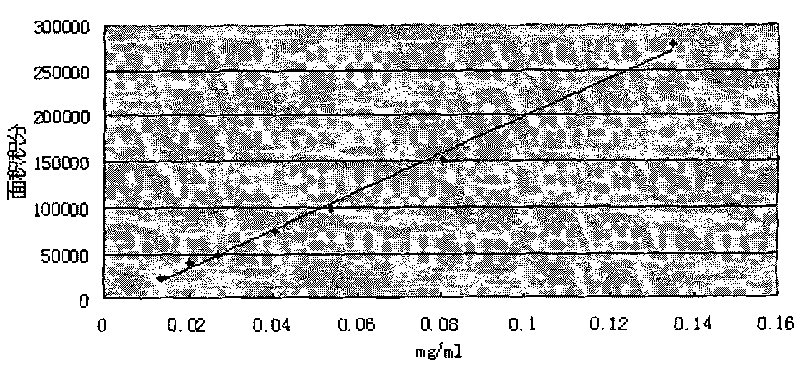
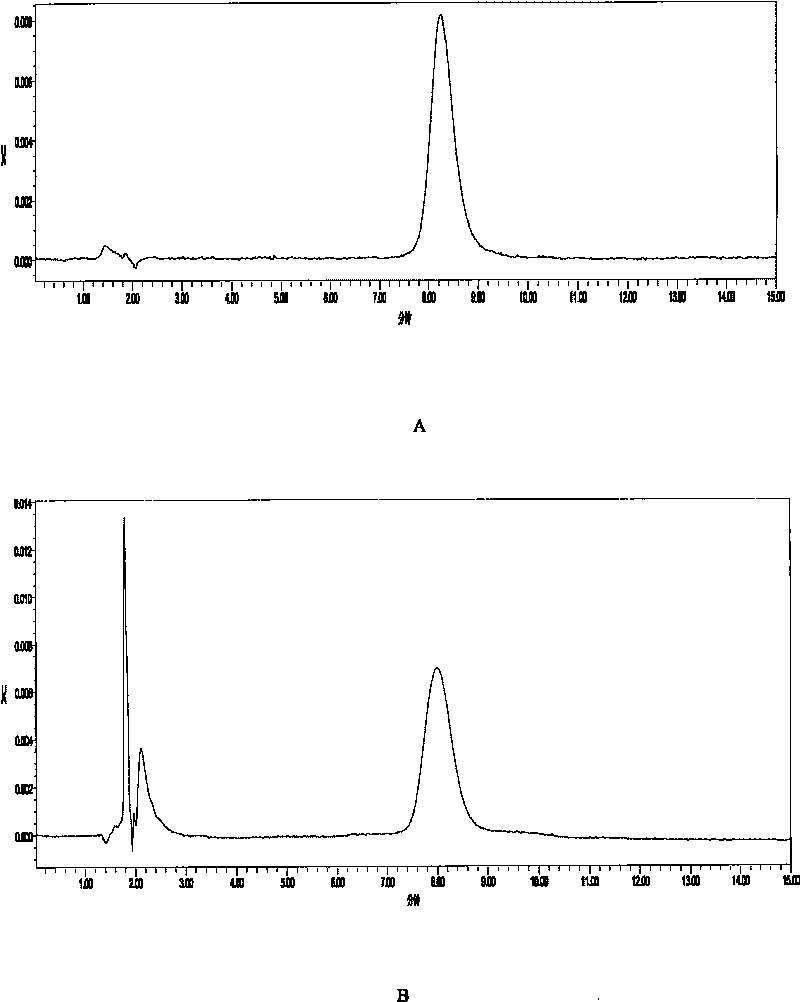
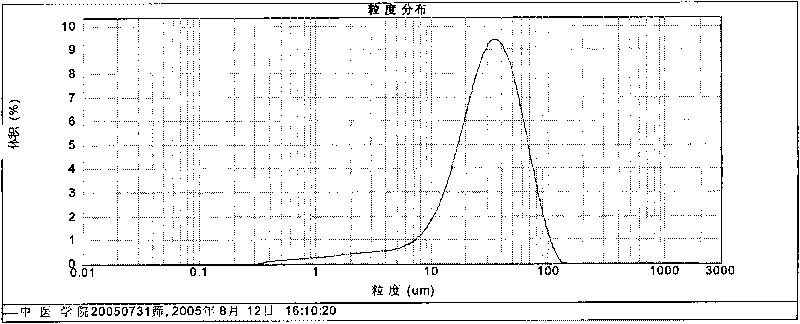
Image
Examples
Embodiment 1
[0144] Embodiment 1: Thymopentin Oral Microspheres
[0145] Thymopentin 0.5kg
[0146] Gelatin 0.4kg
[0147] Lactide-glycolide copolymer 9kg
[0148] Dichloromethane 30L
[0149] Polyvinyl alcohol-124 10kg
[0150] Aprotinin 0.05kg
[0151] Sodium glycocholate 0.2kg;
[0152] Thymopentin, gelatin, aprotinin, and sodium glycocholate were dissolved in 5L of water for injection to make an aqueous solution as the water phase W 1 Dissolve the lactide-glycolide copolymer in 30l of dichloromethane to make a 30% lactide-glycolide copolymer dichloromethane solution as the oil phase 0; use it uniformly at 4°C Pulp machine 10000rpm high-speed vibration and emulsification for 3 minutes, mix the oil and water two phases evenly to make W I / 0 phase, 10kg polyvinyl alcohol-124 was dissolved in 500L water for injection to make a concentration of 2% polyvinyl alcohol-124 aqueous solution, as the water phase W 2 ; Emulsify with a homogenizer at 10,000 rpm for 5 minutes at 4°C, and 1 / 0 ...
Embodiment 2
[0153] Embodiment 2: Thymopentin oral microsphere tablet
[0154] Thymopentin 0.4kg
[0155] Gelatin 0.2kg
[0156] Lactide-glycolide copolymer 4kg
[0157] Dichloromethane 20L
[0158] Polyvinyl Alcohol-124 5kg
[0159] Aprotinin 0.15kg
[0160] Sodium glycocholate 1.5kg;
[0161] Dissolve thymopentin, gelatin, aprotinin, and sodium glycocholate in 2L of water for injection to make an aqueous solution as the water phase W 1 ; Dissolve lactide-glycolide copolymer in 20L dichloromethane to make a 20% lactide-glycolide copolymer dichloromethane solution as oil phase 0; Pulp machine 7000rpm high-speed vibration and emulsification for 2 minutes, mix the oil and water two phases evenly to make W I / 0 phase, dissolve polyvinyl alcohol-124 in 480L water for injection to make a 1% aqueous solution of polyvinyl alcohol-124, and emulsify it with a homogenizer at 14000 rpm for 4 minutes at 3°C, and mix with the previous W 1 / 0 phase is emulsified and included to obtain W 1 / 0 / W ...
Embodiment 3
[0162] Embodiment 3: Thymopentin oral microsphere capsule
[0163] Thymopentin 0.7kg
[0164] Gelatin 1.3kg
[0165] Lactide-glycolide copolymer 16kg
[0166] Dichloromethane 40L
[0167] Polyvinyl alcohol-124 21kg
[0168] Aprotinin 0.03kg
[0169] Sodium glycocholate 0.5kg;
[0170] Dissolve thymopentin, gelatin, aprotinin, and sodium glycocholate in 7L water for injection to make an aqueous solution as the water phase W 1 ; Dissolve lactide-glycolide copolymer in 40L dichloromethane to make a 40% lactide-glycolide copolymer dichloromethane solution as oil phase 0; Pulp machine 14000rpm high-speed vibration and emulsification for 4 minutes, mix the oil and water two phases evenly to make W 1 / 0 phase, polyvinyl alcohol-124 was dissolved in 700L water for injection to make a concentration of 3% polyvinyl alcohol-124 aqueous solution, as the water phase W 2 ; Emulsify at 5°C with a homogenizer at 7000rpm for 7 minutes at high speed, with the former W 1 / 0 phase is emu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com