Method for preparing microspheres with solid-in-oil-in-hydrophilic oil-in-ethanol
An oil-in-oil and ethanol technology, which is applied in the direction of pharmaceutical formulations, medical preparations of non-active ingredients, non-effective ingredients of polymer compounds, etc., can solve the problems of incomplete release and inability to overcome the encapsulation rate, and achieve redispersibility good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
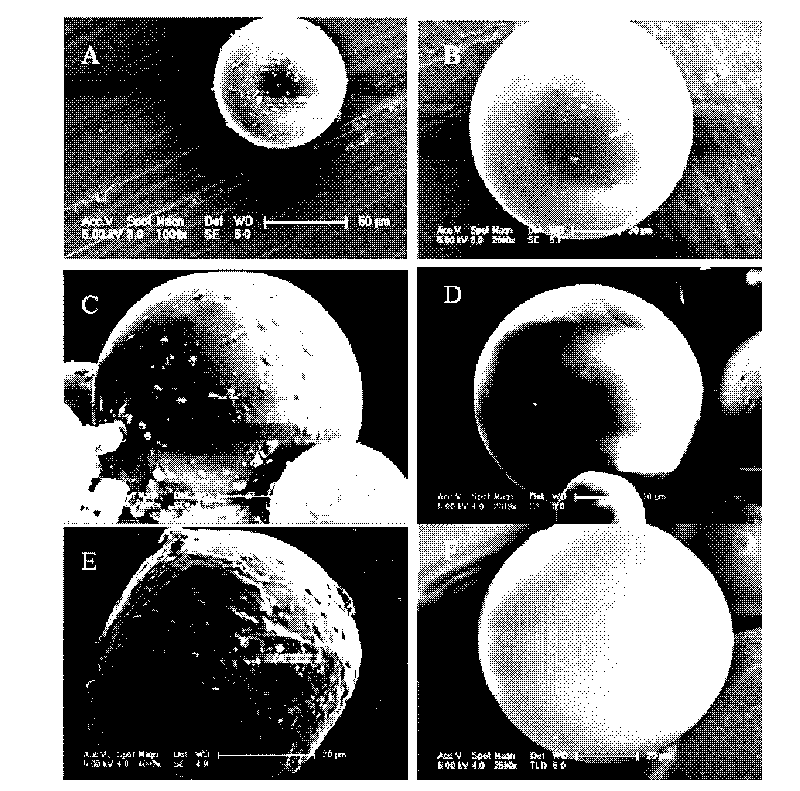
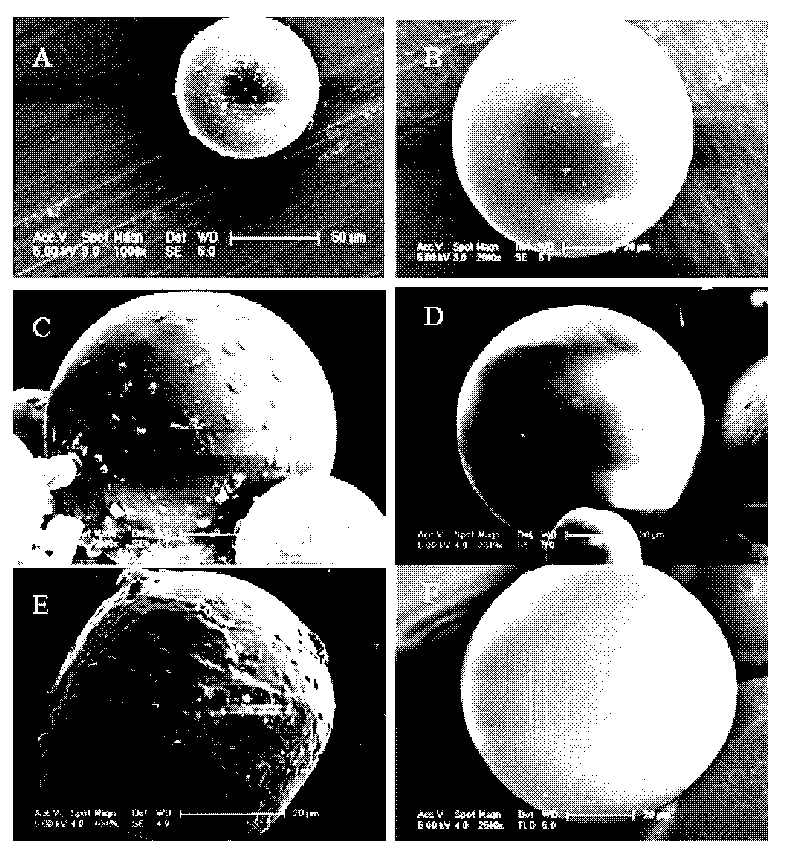
Image
Examples
preparation example Construction
[0037] Preparation of the oil phase (O): the controlled-release or slow-release material is dissolved in an organic solvent to prepare a concentration of 1-50% by weight to form the oil phase (O).
[0038] The preparation of hydrophilic oil phase (hO): formula 1: use surfactant and sodium chloride etc. salt mixed aqueous solution, their weight percentage concentration is respectively 1-10% and 0-10%, accounts for the weight percentage of hydrophilic oil phase 0-40%; ethanol, ethylene glycol, propylene glycol or normal temperature liquid polyethylene glycol is 60-100%; or formula 2: mixed aqueous solution with salts such as surfactant and sodium chloride, and their weight percentage concentrations are respectively 1-10% and 0-10%, accounting for 0-40% by weight of the hydrophilic oil phase; use 0-50% by weight of glycerin and 50-100% of ethanol, ethylene glycol, propylene glycol or normal temperature liquid The polyethylene glycol solution accounts for 60-100% of the hydrophili...
Embodiment 1
[0051] Preparation of polylactic acid-polyglycolic acid (PLGA) microspheres loaded with small molecule drug particles
[0052] (1) According to the small molecule drug particles 0.4mg, 2mg, 1.5mg, 1mg or 0mg (the particle size is 0.2-1μm, 1-5μm, 2-6μm, 2-10μm or 0μm respectively) and the weight percentage concentration is 2 %, 10%, 15%, 20% or 30% PLGA in dichloromethane solution weight ratio of 1:20, 1:30, 1:30, 1:45 or 1:4 equal proportion stirring, vortex or ultrasonic 0.5- Form a uniform suspension in 5 minutes, i.e. a solid in oil (S / O) emulsion (preparing the corresponding drug particles accounts for the percentage of microsphere components: 50%, 40%, 25%, 10% or 0% ;PLGA is 50%, 60%, 75%, 90% or 100%);
[0053] (2) Step (1) is added dropwise to the hydrophilic oil phase (hO) by the emulsion: [The preparation of the hydrophilic oil phase (hO): formula 1: use polyvinyl alcohol (PVA) surfactant and sodium chloride salt Mixed aqueous solution, their weight percentage conc...
Embodiment 2
[0058] Preparation of polylactic acid-polyglycolic acid (PLGA) microspheres loaded with biomacromolecular drug particles
[0059] (1) According to the concentration of 0.4mg, 2mg, 1.5mg, 1mg or 0mg of biomacromolecular drug particles (the particle size is 0.2-1μm, 1-5μm, 2-6μm, 2-10μm or 0μm respectively) and the weight percentage concentration is The weight ratio of 2%, 10%, 15%, 20% or 30% PLGA in dichloromethane solution is 1:20, 1:30, 1:30, 1:45 or 1:4. Stir, vortex or sonicate 0.5 -5 minutes to form a uniform suspension i.e. solid-in-oil (S / O) emulsion (preparing the corresponding drug particles accounts for the percentage of microsphere components are respectively: 50%, 40%, 25%, 10% or 0 %; 50%, 60%, 75%, 90% or 100% for PLGA);
[0060] (2) Step (1) is added dropwise to the hydrophilic oil phase (hO) by the emulsion: [The preparation of the hydrophilic oil phase (hO): formula 1: use polyvinyl alcohol (PVA) surfactant and sodium chloride salt Mixed aqueous solution, th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com