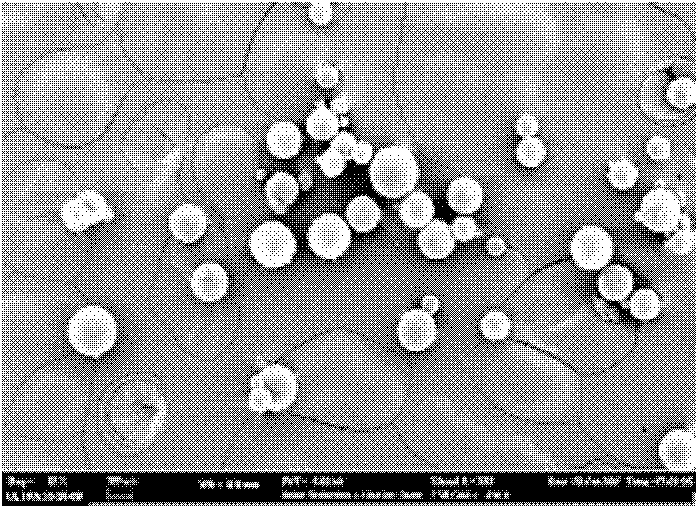
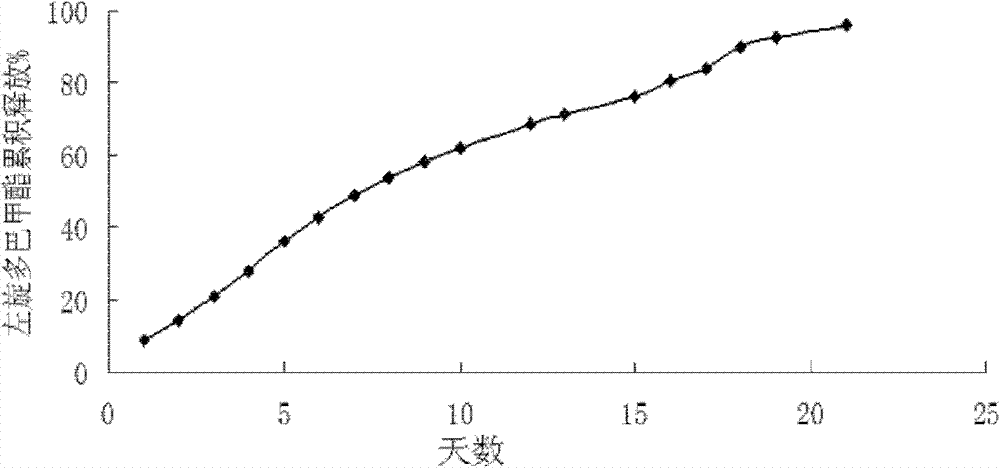
L-dopa methyl ester sustained-release microsphere composite and preparation method thereof
A technology of levodopa methyl ester and slow-release microspheres, which can be used in drug combinations, nervous system diseases, bulk delivery, etc. It can solve the problems of insufficient microspheres for initial sudden release and incomplete release, and achieve a smooth and rounded surface , regular particles, and good redispersibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] ①Preparation of levodopa methyl ester microparticles
[0027] Take 100mg of levodopa methyl ester purchased in the market, first use a microscope to observe whether it is 0.4-10 μm, if not, you can use a pulverizer to crush it into 0.4-5 μm;
[0028] ②Preparation of levodopa methyl ester sustained-release microsphere composition
[0029] (a) The polyglycolic acid-polylactic acid (PLGA, d, l-lactide: 46-52% Mole and glycolide48-54% Mole; molecular weight is 5000-6000) that weighs 495mg is formulated into a concentration of 30% by weight The organic solution of dichloromethane and 5 mg of levodopa methyl ester microparticles obtained in ① were mixed and stirred, vortexed or ultrasonicated for 1-5 minutes to form a uniform suspension, i.e. solid-in-oil (S / O) emulsion; the prepared The theoretical percentage of levodopa methyl ester is 1% sustained-release microspheres.
[0030] (b) Add the emulsion obtained in step (a) to 10 mL of aqueous solution of 1% sodium chloride a...
Embodiment 2
[0035]①Preparation of levodopa methyl ester microparticles
[0036] Take 100mg of levodopa methyl ester purchased in the market, first use a microscope to observe whether it is 0.4-10 μm, if not, you can use a pulverizer to crush it into 0.4-5 μm;
[0037] ②Preparation of levodopa methyl ester sustained-release microsphere composition
[0038] (a) 37.5 mg of polyglycolic acid-polylactic acid (PLGA, d, l-lactide: 46-52% Mole and glycolide48-54% Mole; molecular weight is 5000-6000) is formulated to be 15% by weight The organic solution of dichloromethane and 12.5 mg of levodopa methyl ester microparticles obtained in ① were mixed and stirred, vortexed or ultrasonicated for 1-5 minutes to form a uniform suspension, that is, solid-in-oil (S / O) emulsion; The theoretical percentage content of the prepared levodopa methyl ester is 25% slow-release microspheres.
[0039] (b) Add the emulsion obtained in step (a) to 10 mL of aqueous solution of 5% sodium chloride and 1% polyvinyl alc...
Embodiment 3
[0044] ①Preparation of levodopa methyl ester microparticles
[0045] Take 100mg of levodopa methyl ester purchased in the market, first use a microscope to observe whether it is 0.4-10 μm, if not, you can use a pulverizer to crush it into 0.4-5 μm;
[0046] ②Preparation of levodopa methyl ester sustained-release microsphere composition
[0047] (a) 25 mg of polyglycolic acid-polylactic acid (PLGA, d, l-lactide: 46-52% Mole and glycolide48-54% Mole; molecular weight is 6000) is formulated into a dichloride with a weight percentage concentration of 10%. The organic solution of methane and 25 mg of the levodopa methyl ester particles weighed in ① are mixed and stirred, vortexed or ultrasonicated for 1-5 minutes to form a uniform suspension, that is, solid-in-oil (S / O) emulsion; Theoretical percentage content of dopa methyl ester is 50% sustained-release microspheres.
[0048] (b) adding the emulsion obtained in step (a) to the polyvinyl alcohol (PVA) (molecular weight is 110,00...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com